Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
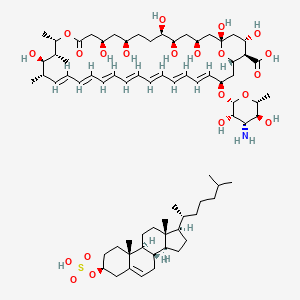

1. Amphotericin B Cholesteryl Sulfate
2. 120895-52-5
3. 774x4698x3
4. Dtxsid50153055
5. Refchem:199573
6. Dtxcid8075546
7. Unii-774x4698x3
8. Abcd
9. Schembl3669433
10. Hy-125606
11. Amphotericin B Cholesteryl Sulfate [vandf]
12. Q27266548
13. Amphotericin B Compd With Cholesteryl Sulfate [mi]
14. Amphotericin B Compd With (3.beta.)-cholest-5-en-3-yl Hydrogen Sulfate (1:1
| Molecular Weight | 1390.8 g/mol |
|---|---|
| Molecular Formula | C74H119NO21S |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 22 |
| Rotatable Bond Count | 10 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 392 |
| Heavy Atom Count | 97 |
| Formal Charge | 0 |
| Complexity | 2480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 27 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 7 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Amebicides
Agents which are destructive to amebae, especially the parasitic species causing AMEBIASIS in man and animal.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues.
Antiprotozoal Agents
Substances that are destructive to protozoans.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA.
ABOUT THIS PAGE

