
Synopsis
Synopsis
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Alprazolan
2. Alprox
3. Apo Alpraz
4. Apo-alpraz
5. Cassadan
6. D-65mt
7. D65mt
8. Esparon
9. Kalma
10. Novo Alprazol
11. Novo-alprazol
12. Nu Alpraz
13. Nu-alpraz
14. Ralozam
15. Tafil
16. Trankimazin
17. U-31,889
18. U31,889
19. Xanax
1. Xanax
2. 28981-97-7
3. Trankimazin
4. Niravam
5. Tafil
6. Tranquinal
7. Alplax
8. Constan
9. Frontal
10. Solanax
11. Xanor
12. Apo-alpraz
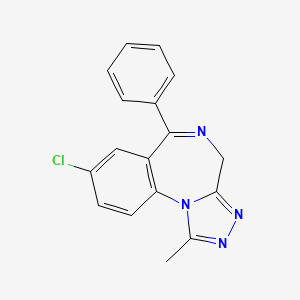
13. 8-chloro-1-methyl-6-phenyl-4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
14. Xanax Xr
15. Alcelam
16. Anpress
17. Relaxol
18. Tricalma
19. Valeans
20. Xanagis
21. Zolarem
22. Alpaz
23. Alpram
24. Alzam
25. Panix
26. Prinox
27. Zoldac
28. Zotran
29. Alprazolam Intensol
30. Tus-1
31. Xanolam
32. Zolam
33. Zopax
34. Zopic
35. Tafil D
36. Xanax Ts
37. D 65mt
38. Alprazolam Civ
39. 8-chloro-1-methyl-6-phenyl-4h-s-triazolo(4,3-a)(1,4)benzodiazepine
40. U-31,889
41. Cassadan
42. Esparon
43. Neurol
44. U 31889
45. U-31889
46. Alpronax
47. Alzolam
48. Bestrol
49. Ralozam
50. Alprox
51. Restyl
52. Yu55mq3izy
53. Chembl661
54. Nsc-760140
55. Novo-alprazol
56. 8-chloro-1-methyl-6-phenyl-4h-s-triazolo[4,3-a][1,4]benzodiazepine
57. Nu-alpraz
58. Chebi:2611
59. Alprazolamum
60. Pharnax
61. Prazolan
62. Tensivan
63. Zacetin
64. Zanapam
65. Alprax
66. Ksalol
67. Mialin
68. Prazam
69. Unilan
70. Zoldax
71. Algad
72. Alzon
73. Helex
74. Zaxan
75. Zenax
76. Gen-alprazolan
77. 4h-s-triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-1-methyl-6-phenyl-
78. 4h-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-1-methyl-6-phenyl-
79. 4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine, 8-chloro-1-methyl-6-phenyl-
80. Ncgc00159466-02
81. Alprazolamum [inn-latin]
82. U 31,889
83. Tus 1
84. Panistat
85. Alprazolam Extended Release Tablets
86. Staccato-alprazolam
87. Smr000149316
88. Xanax (tn)
89. Hsdb 7207
90. Einecs 249-349-2
91. Unii-yu55mq3izy
92. Az-002
93. Tgar01p
94. Brn 1223125
95. Dea No. 2882
96. 08h
97. Alprazolam [usan:usp:inn:ban:jan]
98. Ap-1002
99. 3u5j
100. Alprazolam [mi]
101. Alprazolam [inn]
102. Alprazolam [jan]
103. Alprazolam [hsdb]
104. Alprazolam [usan]
105. Dsstox_cid_2577
106. Alprazolam [vandf]
107. Alprazolam [mart.]
108. Schembl8398
109. Alprazolam [who-dd]
110. Dsstox_rid_76639
111. Bidd:pxr0150
112. Dsstox_gsid_22577
113. Zinc903
114. Mls000559000
115. Mls000759485
116. Mls001423979
117. Bidd:gt0475
118. Alprazolam (jp17/usp/inn)
119. Gtpl7111
120. Alprazolam [ep Impurity]
121. Alprazolam [orange Book]
122. Dtxsid4022577
123. Alprazolam [ep Monograph]
124. Alprazolam [usp Monograph]
125. Hms2051a10
126. Hms3393a10
127. Pharmakon1600-01502395
128. Alprazolam 0.1 Mg/ml In Methanol
129. Alprazolam 1.0 Mg/ml In Methanol
130. Bcp28608
131. Tox21_111692
132. Bbl028160
133. Bdbm50001728
134. Nsc760140
135. Stk590494
136. Akos005066050
137. Ccg-100855
138. Db00404
139. Nc00105
140. Nsc 760140
141. 4h-(1,2,4)triazolo(4,3-alpha)(1,4)benzodiazepine, 8-chloro-1-methyl-6-phenyl-
142. Ncgc00159466-03
143. 51339-48-1
144. Ac-18721
145. Cas-28981-97-7
146. C06817
147. D00225
148. 981a977
149. A819702
150. Q319877
151. W-107015
152. Brd-k32398298-001-01-4
153. Alprazolam, European Pharmacopoeia (ep) Reference Standard
154. 8-chloro-1-methyl-6-phenyl-4h-s-triazolo [4,3-a] [1,4] Benzodiazepine
155. 8-chloro-1-methyl-6-phenyl-4h-s-triazolo [4,3-a][1,4] Benzodiazepine
156. 8-chloro-6-phenyl-1-methyl-4h-s-triazolo-[4,3-a][1,4]benzodiazepine
157. (z)-8-chloro-1-methyl-6-phenyl-4h-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine
158. 4h-(1,2,4)triazolo(4,3-.alpha.)(1,4)benzodiazepine, 8-chloro-1-methyl-6-phenyl-
159. 8-chloranyl-1-methyl-6-phenyl-4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
160. 8-chloro-1-methyl-6-phenyl-4h-s-triazolo(4,3-.alpha.)(1,4)benzodiazepine
161. Alprazolam Solution, 1 Mg/ml In Methanol, Analytical Standard, For Drug Analysis
162. Alprazolam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
163. 12-chloro-3-methyl-9-phenyl-2,4,5,8-tetraazatricyclo[8.4.0.0^{2,6}]tetradeca-1(10),3,5,8,11,13-hexaene
164. 1246182-61-5
| Molecular Weight | 308.8 g/mol |
|---|---|
| Molecular Formula | C17H13ClN4 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 308.0828741 g/mol |
| Monoisotopic Mass | 308.0828741 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 434 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Alprazolam |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Alprazolamextended-release tabletscontain alprazolam USP, which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, extended release; Tablet, orally disintegrating; Tablet; Concentrate |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 2mg; 1mg; 0.25mg; 3mg |
| Market Status | Prescription |
| Company | Corepharma; Vintage Pharms; Anchen Pharms; Mylan Pharms; Amneal Pharms Ny; Actavis Elizabeth; Ani Pharms; Aurobindo Pharma Usa; Actavis Labs Fl; Apotex; Vintage; Dava Intl; Zydus Pharms Usa; Sandoz; Sun Pharma Global; Par Pharm; Mylan; Impax Labs; Roxane |
| 2 of 10 | |
|---|---|
| Drug Name | Azor |
| PubMed Health | Amlodipine/Olmesartan (By mouth) |
| Drug Classes | Antihypertensive |
| Active Ingredient | olmesartan medoxomil; Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base; 40mg; 20mg |
| Market Status | Prescription |
| Company | Daiichi Sankyo |
| 3 of 10 | |
|---|---|
| Drug Name | Niravam |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | NIRAVAM (alprazolam orally disintegrating tablets) contains alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. NIRAVAM is an orally administered formulation of alprazolam which rapidl... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 2mg |
| Market Status | Prescription |
| Company | Ucb |
| 4 of 10 | |
|---|---|
| Drug Name | Xanax |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | XANAX XR Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [1,4] benzodiazepine. The m... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 2mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 5 of 10 | |
|---|---|
| Drug Name | Xanax xr |
| Drug Label | XANAX XR Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [1,4] benzodiazepine. The m... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 0.5mg; 1mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 6 of 10 | |
|---|---|
| Drug Name | Alprazolam |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Alprazolamextended-release tabletscontain alprazolam USP, which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, extended release; Tablet, orally disintegrating; Tablet; Concentrate |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 2mg; 1mg; 0.25mg; 3mg |
| Market Status | Prescription |
| Company | Corepharma; Vintage Pharms; Anchen Pharms; Mylan Pharms; Amneal Pharms Ny; Actavis Elizabeth; Ani Pharms; Aurobindo Pharma Usa; Actavis Labs Fl; Apotex; Vintage; Dava Intl; Zydus Pharms Usa; Sandoz; Sun Pharma Global; Par Pharm; Mylan; Impax Labs; Roxane |
| 7 of 10 | |
|---|---|
| Drug Name | Azor |
| PubMed Health | Amlodipine/Olmesartan (By mouth) |
| Drug Classes | Antihypertensive |
| Active Ingredient | olmesartan medoxomil; Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base; 40mg; 20mg |
| Market Status | Prescription |
| Company | Daiichi Sankyo |
| 8 of 10 | |
|---|---|
| Drug Name | Niravam |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | NIRAVAM (alprazolam orally disintegrating tablets) contains alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. NIRAVAM is an orally administered formulation of alprazolam which rapidl... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 2mg |
| Market Status | Prescription |
| Company | Ucb |
| 9 of 10 | |
|---|---|
| Drug Name | Xanax |
| PubMed Health | Alprazolam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | XANAX XR Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [1,4] benzodiazepine. The m... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 2mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 10 of 10 | |
|---|---|
| Drug Name | Xanax xr |
| Drug Label | XANAX XR Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-] [1,4] benzodiazepine. The m... |
| Active Ingredient | Alprazolam |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 0.5mg; 1mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Anti-Anxiety Agents; GABA Modulators; Hypnotics and Sedatives
National Library of Medicine's Medical Subject Headings. Alprazolam. Online file (MeSH, 2016). Available from, as of October 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
Xanax Tablets (alprazolam) are indicated for the management of anxiety disorder (a condition corresponding most closely to the APA Diagnostic and Statistical Manual (DSM-IIIR) diagnosis of generalized anxiety disorder) or the short-term relief of symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Xanax is also indicated for the treatment of panic disorder, with or without agoraphobia. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
VET: /Alprazolam/ may be useful in cats to treat anxiety disorders and unlike oral diazepam in cats, has not been implicated in causing liver failure.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 45
VET: Alprazolam may be useful for adjunctive therapy in anxious, aggressive dogs or in those demonstrating panic reactions. It is mose effective when used in advance of a triggering event. (NOTE: Some clinicians believe that benzodiazepines are contraindicated in aggressive dogs as anxiety may actually restrain the animal from aggressive tendencies.)
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 45
Episodes of mania and hypomania have been reported in depressed patients receiving alprazolam.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2381
Pregnancy risk category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Benzodiazepines can potentially cause fetal harm when administered to pregnant women. If Xanax is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Because of experience with other members of the benzodiazepine class, Xanax is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency, their use during the first trimester should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Safety and efficacy of alprazolam have not been established in children younger than 18 years of age.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2381
For more Drug Warnings (Complete) data for ALPRAZOLAM (16 total), please visit the HSDB record page.
Alprazolam is indicated for the acute treatment of generalized anxiety disorder in adults. Alprazolam is also indicated, either as a standard or extended-release formulation, for the treatment of panic disorder with or without agoraphobia in adults. Alprazolam may also be prescribed off-label for insomnia, premenstrual syndrome, and depression.
FDA Label
Alprazolam is a benzodiazepine that binds -aminobutyric acid (GABA) type-A receptors (GABAARs) to enhance their inhibitory effect on neurotransmission, specifically in the brain. Concomitant use with opioids may result in profound sedation, respiratory depression, coma, and death; patients taking benzodiazepines and opioids concurrently may require lower doses of one or both medications, depending on their clinical situation. Patients with pre-existing impaired respiratory function are at increased risk of adverse effects including death during treatment with benzodiazepines. In addition, due to its CNS depressant effects, patients taking alprazolam should avoid operating heavy machinery or driving and should avoid other CNS depressants such as alcohol. As with other benzodiazepines, alprazolam carries a risk of abuse, misuse, and addiction, which is higher in predisposed individuals and may require strict monitoring. Cessation of therapy may result in acute or protracted withdrawal symptoms, which may be life-threatening; the patient dose should be gradually tapered whenever discontinuation or reduced dosage are necessary. Newborns born to mothers using alprazolam later in pregnancy may suffer from sedation and withdrawal symptoms. As CYP3A is required for the initial step in alprazolam metabolism, alprazolam is contraindicated in patients taking strong CYP3A inhibitors, such as ketoconazole and itraconazole; milder CYP3A inhibitors still necessitate alprazolam dosage adjustments. Lastly, benzodiazepines may have negative effects, such as panic disorders, increased suicide incidence, and episodes of mania/hypomania, in patients suffering from depression.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
N05BA12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA12 - Alprazolam
Absorption
Alprazolam administered orally is rapidly absorbed in the gastrointestinal tract, reaching Cmax in about 1.8 (1-2) hours. Absorption is high, resulting in an oral bioavailability of 84-91%. A 1 mg oral dose results in a Cmax of 12-22 g/L. The extended-release formulation of alprazolam (XANAX XR) has similar absorption, bioavailability, and pharmacokinetics as the standard release, with the exception that the Tmax is ~10 hours compared to 1-2 hours. Temporal dosing alters these parameters, with Cmax increasing by 30% and Tmax decreasing by one hour when dosed at night as opposed to in the morning. Food has an effect on alprazolam absorption; a high-fat meal up to two hours before dosing increases the Cmax by ~25% and either a reduction (food consumed immediately prior to dosing) or increase (food consumed after dosing) of ~1/3 in Tmax. Neither the AUC nor half-life are appreciably affected by eating.
Route of Elimination
Alprazolam is mainly eliminated in the urine. A large portion of the dose is eliminated as unmetabolized alprazolam. <10% of the dose is eliminated as alpha-hydroxy-alprazolam and 4-hydroxy-alprazolam.
Volume of Distribution
Alprazolam has a volume of distribution following oral administration of 0.8-1.3L/kg. Alprazolam crosses the blood-brain barrier.
Clearance
A 0.8 mg oral dose of alprazolam had a clearance of 0.90 0.21 mL/min/kg, which increased to 2.13 0.54 mL/min/kg when coadministered with the strong CYP3A4 inducer carbamazepine. Other studies have demonstrated a clearance of 0.70-1.5mL/min/kg.
In vitro, alprazolam is bound (80 percent) to human serum protein. Serum albumin accounts for the majority of the binding.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Alprazolam and its metabolites are excreted primarily in the urine.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Following oral administration, alprazolam is readily absorbed. Peak concentrations in the plasma occur in 1 to 2 hours following administration. Plasma levels are proportionate to the dose given; over the dose range of 0.5 to 3.0 mg, peak levels of 8.0 to 37 ng/mL were observed.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
The newborn of a mother reporting alprazolam use during pregnancy presented with respiratory distress and clinical features consistent with neonatal withdrawal syndrome or neonatal sepsis of vertical transmission. Alprazolam and its main metabolite (alpha-hydroxyalprazolam) were detected in cord serum, neonatal urine and also in neonatal hair, meconium and placenta, accounting for both acute and chronic exposure to this benzodiazepine during intrauterine life. ...
PMID:17453885 Garcia-Algar O et al; Clin Toxicol (Phila) 45 (3): 295-8 (2007)
Alprazolam is metabolized to less effective metabolites by various CYPs including CYP3A4, CYP3A5, CYP3A7, and CYP2C9. The majority of alprazolam metabolism is mediated by hydroxylation via CYP3As. 4-hydroxyalprazolam has 20% the binding affinity of the parent drug, alpha-hydroxyalprazolam has 66% the affinity, and the benzophenone metabolite has <1% the affinity.
Alprazolam, an anti-anxiety agent, is metabolized in rat and human liver by P4503A1 and P4503A4 respectively, to 4-hydroxy alprazolam (4-OHALP, pharmacologically less active) and alpha-hydroxy alprazolam (alpha-OHALP, pharmacologically more active). We examined P450 mediated metabolism of alprazolam by rat and human brain microsomes and observed that the relative amount of alpha-OHALP formed in brain was higher than liver. This biotransformation was mediated by a P450 isoform belonging to P4503A subfamily, which is constitutively expressed in neuronal cells in rat and human brain. The formation of larger amounts of alpha-OHALP in neurons points to local modulation of pharmacological activity in brain, at the site of action of the anti-anxiety drug. Since hydroxy metabolites of alprazolam are hydrophilic and not easily cleared through blood-CSF barrier, alpha-OHALP would potentially have a longer half-life in brain.
PMID:12196913 Pai HV et al; Pharmacogenomics J 2 (4): 243-58 (2002)
Alprazolam is extensively metabolized in humans, primarily by cytochrome P450 3A4 (CYP3A4), to two major metabolites in the plasma: 4-hydroxyalprazolam and a-hydroxyalprazolam. A benzophenone derived from alprazolam is also found in humans. Their half-lives appear to be similar to that of alprazolam. The plasma concentrations of 4-hydroxyalprazolam and a-hydroxyalprazolam relative to unchanged alprazolam concentration were always less than 4%. The reported relative potencies in benzodiazepine receptor binding experiments and in animal models of induced seizure inhibition are 0.20 and 0.66, respectively, for 4-hydroxyalprazolam and a-hydroxyalprazolam. Such low concentrations and the lesser potencies of 4-hydroxyalprazolam and a-hydroxyalprazolam suggest that they are unlikely to contribute much to the pharmacological effects of alprazolam. The benzophenone metabolite is essentially inactive.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Alprazolam has known human metabolites that include 4-Hydroxyalprazolam and Beta-Hydroxyalprazolam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Alprazolam has a mean plasma elimination half-life of 11.2 hours in healthy patients (range 6.3-26.9 hours). The mean half-life is 16.3 hours (range 9.0-26.9 hours) in the elderly, 21.8 hours (range 9.9-40.4 hours) in obese patients, and 19.7 hours (range 5.8-65.3 hours) in patients with alcoholic liver disease. The half-life is 25% higher in Asian patients compared to Caucasians. Other studies have shown the half-life to be 9-16h. The extended-release formulation has a half-life of 10.7-15.8 hours in healthy adult patients.
Using a specific assay methodology, the mean plasma elimination half-life of alprazolam has been found to be about 11.2 hours (range: 6.3-26.9 hours) in healthy adults.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Neurotransmission relies on excitatory and inhibitory signalling. -aminobutyric acid (GABA) type-A receptors (GABAARs) are members of the pentameric ligand-gated ion channel (PLGIC) superfamily located synaptically and perisynaptically to mediate phasic inhibition and extrasynaptically to mediate tonic inhibition. GABAARs comprise a variety of subunits from a homologous family whose members are named based on sequence identity as one of 1-6, 1-3, 1-3, , , , , and 1-3. Each subunit possesses an extracellular (ECD), transmembrane (TMD), and intracellular (ICD) domain; inter-subunit interfaces are the primary points of neurotransmitter and modulator binding, described by coordination of the principal (+) and complementary (-) sites in each subunit. Binding of GABA to GABAARs induces pore opening, rapid flow of chloride ions, and synaptic hyperpolarization, which in turn manifests as an inhibitory signal. The most prevalent GABAARs _in vivo_ are the 122 receptors, which contain both GABA (+/-) and benzodiazepine (BZD, +/-) binding sites in the intersubunit interfaces of the relevant subunits. In general, any receptors containing an x/z interface, where x = 1-3,5 and z = 1-3, have potential high-affinity BZD binding sites, although small sequence differences between subunits may alter binding affinity to individual molecules. The 4 and 6 subunits, in which an otherwise conserved histidine is replaced by arginine, do not bind traditional BZD ligands such as diazepam and hence are considered "diazepam-insensitive". GABA binding results in a series of conformational changes in the ECDs of GABAAR subunits, "locking" each to its neighbouring - interface. The binding of alprazolam in the high-affinity BZD site stabilizes the +/- interface and facilitates the conformational changes that lead to pore opening, hence functioning as a positive allosteric modulator. The exact manner in which GABAAR allosteric modulation mediates the therapeutic and unwanted effects of benzodiazepines remains unclear. Earlier studies suggested that the primary factor was the subunit composition, with 1-containing receptors mediating the sedative effects, 2/3-containing receptors the anxiolytic effects, and 5-containing receptors the memory effects of benzodiazepines. More recent studies suggest a more complex set of factors including subunit composition, physiological location, neuronal circuit, and nerve cell type. To further complicate matters, there may be up to five distinct BZD binding sites on GABAARs, with site 1 corresponding to the classical high-affinity +/- interface. The effects of binding at sites 2-4 are not fully understood and likely impart greater complexity to benzodiazepine pharmacological action.
In animals, benzodiazepines protect against seizures induced by electrical stimulation and by pentylenetetrazol; benzodiazepines appear to act, at least partly, by augmenting presynaptic inhibition. The drugs suppress the spread of seizure activity but do not abolish the abnormal discharge from a focus in experimental models of epilepsy. In usual doses, benzodiazepines appear to have very little effect on the autonomic nervous system, respiration, or the cardiovascular system. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
CNS agents of the 1,4 benzodiazepine class presumably exert their effects by binding at stereo specific receptors at several sites within the central nervous system. Their exact mechanism of action is unknown. Clinically, all benzodiazepines cause a dose-related central nervous system depressant activity varying from mild impairment of task performance to hypnosis.
NIH; DailyMed. Current Medication Information for Xanax (Alprazolam) Tablet (Updated: October 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=388e249d-b9b6-44c3-9f8f-880eced0239f
Anxiolytic and possibly paradoxical CNS stimulatory effects of benzodiazepines are postulated to result from release of previously suppressed responses (disinhibition). After usual doses of benzodiazepines for several days, the drugs cause a moderate decrease in rapid eye movement (REM) sleep. REM rebound does not occur when the drugs are withdrawn. Stage 3 and 4 sleep are markedly reduced by usual doses of the drugs; the clinical importance of these sleep stage alterations has not been established. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
Benzodiazepines appear to produce skeletal muscle relaxation predominantly by inhibiting spinal polysynaptic afferent pathways, but the drugs may also inhibit monosynaptic afferent pathways. The drugs may inhibit monosynaptic and polysynaptic reflexes by acting as inhibitory neuronal transmitters or by blocking exitatory synaptic transmission. The drugs may also directly depress motor nerve and muscle function. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
83
PharmaCompass offers a list of Alprazolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Alprazolam manufacturer or Alprazolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Alprazolam manufacturer or Alprazolam supplier.
PharmaCompass also assists you with knowing the Alprazolam API Price utilized in the formulation of products. Alprazolam API Price is not always fixed or binding as the Alprazolam Price is obtained through a variety of data sources. The Alprazolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alzam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alzam, including repackagers and relabelers. The FDA regulates Alzam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alzam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Alzam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Alzam supplier is an individual or a company that provides Alzam active pharmaceutical ingredient (API) or Alzam finished formulations upon request. The Alzam suppliers may include Alzam API manufacturers, exporters, distributors and traders.
click here to find a list of Alzam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Alzam DMF (Drug Master File) is a document detailing the whole manufacturing process of Alzam active pharmaceutical ingredient (API) in detail. Different forms of Alzam DMFs exist exist since differing nations have different regulations, such as Alzam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Alzam DMF submitted to regulatory agencies in the US is known as a USDMF. Alzam USDMF includes data on Alzam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Alzam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Alzam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Alzam Drug Master File in Japan (Alzam JDMF) empowers Alzam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Alzam JDMF during the approval evaluation for pharmaceutical products. At the time of Alzam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Alzam suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Alzam Drug Master File in Korea (Alzam KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Alzam. The MFDS reviews the Alzam KDMF as part of the drug registration process and uses the information provided in the Alzam KDMF to evaluate the safety and efficacy of the drug.
After submitting a Alzam KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Alzam API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Alzam suppliers with KDMF on PharmaCompass.
A Alzam CEP of the European Pharmacopoeia monograph is often referred to as a Alzam Certificate of Suitability (COS). The purpose of a Alzam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Alzam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Alzam to their clients by showing that a Alzam CEP has been issued for it. The manufacturer submits a Alzam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Alzam CEP holder for the record. Additionally, the data presented in the Alzam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Alzam DMF.
A Alzam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Alzam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Alzam suppliers with CEP (COS) on PharmaCompass.
A Alzam written confirmation (Alzam WC) is an official document issued by a regulatory agency to a Alzam manufacturer, verifying that the manufacturing facility of a Alzam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Alzam APIs or Alzam finished pharmaceutical products to another nation, regulatory agencies frequently require a Alzam WC (written confirmation) as part of the regulatory process.
click here to find a list of Alzam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Alzam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Alzam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Alzam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Alzam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Alzam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Alzam suppliers with NDC on PharmaCompass.
Alzam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alzam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alzam GMP manufacturer or Alzam GMP API supplier for your needs.
A Alzam CoA (Certificate of Analysis) is a formal document that attests to Alzam's compliance with Alzam specifications and serves as a tool for batch-level quality control.
Alzam CoA mostly includes findings from lab analyses of a specific batch. For each Alzam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alzam may be tested according to a variety of international standards, such as European Pharmacopoeia (Alzam EP), Alzam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alzam USP).

