Phispers On Air
Click & Listen

By PharmaCompass
2025-12-04
Impressions: 117 Article || 1 Video || 20 Listen
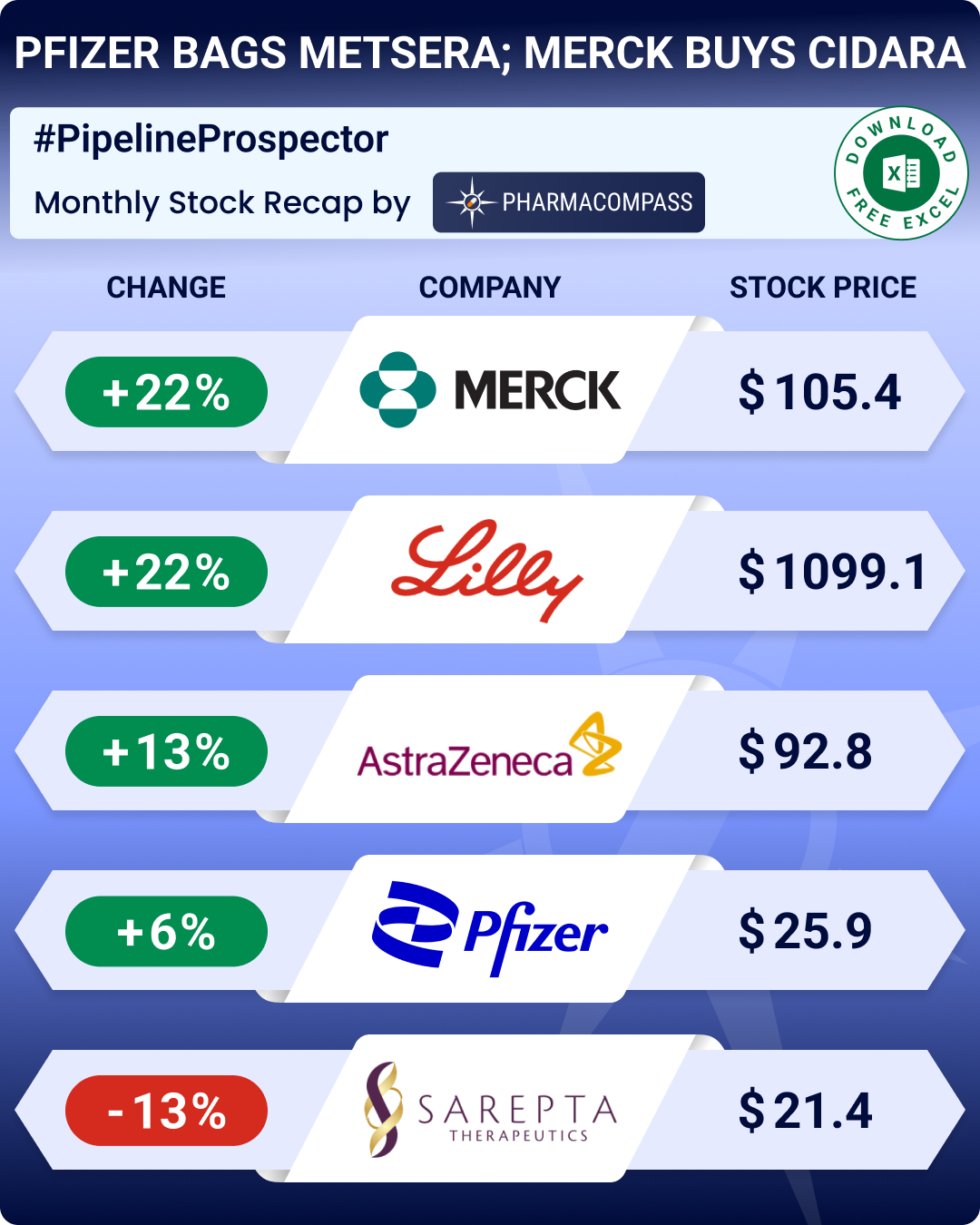
In Phispers this week, Eli Lilly reduced prices of its obesity drug Zepbound sold through LillyDirect, aiming to expand access amid skyrocketing demand for its weight-loss medicine.
A leaked memo of the US Food and Drug Administration (FDA) suggests that Covid-19 vaccines may have caused deaths of 10 children. The agency is now planning a stricter approach to vaccine approvals. Reacting to the memo, a dozen former FDA commissioners have cautioned that a stricter direction on vaccine approvals may slow access to lifesaving immunizations in the US.
The United States and the United Kingdom have finalized a trade agreement that eliminates US tariffs on British pharmaceuticals and medical technology.
A US federal judge refused to dismiss major portions of a US$ 6.7 billion lawsuit filed by Celgene shareholders that accuses Bristol Myers Squibb (BMS) of deliberately delaying FDA approvals tied to its acquisition of Celgene.
In clinical trials, Belite Bio reported a phase 3 win for its oral therapy tinlarebant in treating Stargardt disease (a genetic eye disorder), setting up a potential FDA submission next year.
Meanwhile, FDA witnessed leadership change yet again — just weeks after being appointed head of FDA’s Center for Drug Evaluation and Research (CDER), there is news that Richard Pazdur is retiring. FDA has named Tracy Beth Hoeg as the acting director of CDER.
Weeks after signing deal with Trump, Lilly cuts price of Zepbound sold on its online platform
Just weeks after US President Donald Trump inked a deal with Eli Lilly and Novo Nordisk to make their GLP-1 drugs easier for Americans to procure and afford, Lilly has lowered the price of its obesity drug Zepbound (tirzepatide) for patients who buy the single-dose vials through its LillyDirect platform. This step will make Zepbound more affordable to people in the US.
Zepbound 2.5mg will now cost US$ 299 per month through LillyDirect, as opposed to US$ 349 earlier. Similarly, the price of 5mg dose has been reduced from US$ 499 per month to US$ 399. Both these prices are applicable to patients with prescriptions.
WHO backs use of GLP-1 therapies for obesity: The World Health Organization issued its first-ever guidance on GLP-1 therapies for obesity, conditionally recommending the drugs as part of long-term treatment for adults, alongside healthy diet and exercise.
‘Leaked memo’ links Covid-19 vaccines to child deaths; former FDA chiefs react
A leaked memo from Vinay Prasad, FDA’s chief medical and scientific officer, triggered debate both inside and outside the agency. In the memo, Prasad has alleged that Covid-19 vaccines possibly contributed to the deaths of at least 10 children, who died of heart inflammation.
According to a Reuters news report, the memo said the deaths are related to Covid vaccination. “This is a profound revelation. For the first time, the USFDA will acknowledge that Covid-19 vaccines have killed American children,” the memo said. FDA is now planning a stricter approach to vaccine approvals.
Reacting to the memo, a dozen former FDA commissioners cautioned that a stricter new direction on vaccine approvals may slow access to lifesaving immunizations in the US. In an article published in the New England Journal of Medicine, the former officials said they are “deeply concerned” about vaccine policies outlined in the leaked memo.
The memo comes at a time when the US Health Secretary Robert F Kennedy Jr, a longtime vaccine critic now overseeing US health agencies, has already limited access to Covid vaccines.
Judge refuses to dismiss US$ 6.7 billion lawsuit filed by Celgene shareholders
A US federal judge has refused to dismiss major parts of a US$ 6.7 billion lawsuit, accusing Bristol Myers Squibb (BMS) of intentionally slowing down FDA approvals for three Celgene drugs in order to avoid making payouts tied to its 2019 acquisition of Celgene. The case centers on contingent value rights (CVRs) held by shareholders. BMS had promised to pay former Celgene shareholders an extra US$ 9 per share if BMS had secured timely FDA approvals for Breyanzi (lisocabtagene maraleucel), Ozanimod and Ide-cel (idecabtagene vicleucel). Breyanzi ultimately received FDA approval five weeks after the deadline, thereby denying the shareholders of the payout.
US finalizes trade deal with UK, eliminates tariffs on pharmaceuticals, medtech
The United States and the United Kingdom have finalized a trade agreement that eliminates US tariffs on British pharmaceutical products and medical technology. In September, the US President Donald Trump had said he would raise tariffs to 100 percent on branded drug imports.
Under the agreement, the UK will pay more for drugs through the NHS in return for a guarantee that America’s import tariff on medicines made in the UK will remain zero for three years. The UK exported medicines worth £11.1 billion (US$ 14.81 billion) to the US during October 1, 2024 to September 31, 2025, accounting for 17.4 percent of its total exports.
Belite Bio’s oral drug slows eye damage in Stargardt disease in phase 3 trial
Belite Bio has reported positive phase 3 results for tinlarebant, an oral treatment for Stargardt disease type 1 (STGD1), a rare inherited condition that causes progressive central vision loss in children and young adults. Belite plans to seek FDA approval in the first half of next year. If cleared, tinlarebant could become the first approved treatment for STGD1 in the US.
Tracy Beth Hoeg appointed acting director of CDER after Pazdur’s retirement
Just weeks after being appointed to lead the FDA’s Center for Drug Evaluation and Research (CDER), Richard Pazdur is preparing to retire, marking another major leadership shake-up at the agency. Pazdur, who has spent 26 years at the FDA, stepped into the role in November following the forced resignation of his predecessor George Tidmarsh.
Meanwhile, FDA has named Tracy Beth Hoeg as acting director of the CDER. Hoeg, a physician and epidemiologist who has publicly challenged several American Covid-19 policies, is considered a close ally of FDA Commissioner Marty Makary.
FDA proposes fee cuts to boost US-based drug development: FDA has proposed lowering user fees for early-stage biopharma companies that conduct phase 1 trials in the US, while imposing higher fees on those that carry out development abroad, according to a summary of Prescription Drug User Fee Act (PDUFA) reauthorization negotiations. FDA Commissioner Marty Makary has framed the fee proposal as part of an “America-first agenda,” arguing it will help restore the US’s lead in drug innovation and counter China’s growing dominance in early-stage trials.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Phispers Infographic by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”