By PharmaCompass
2019-06-27
Impressions: 19535
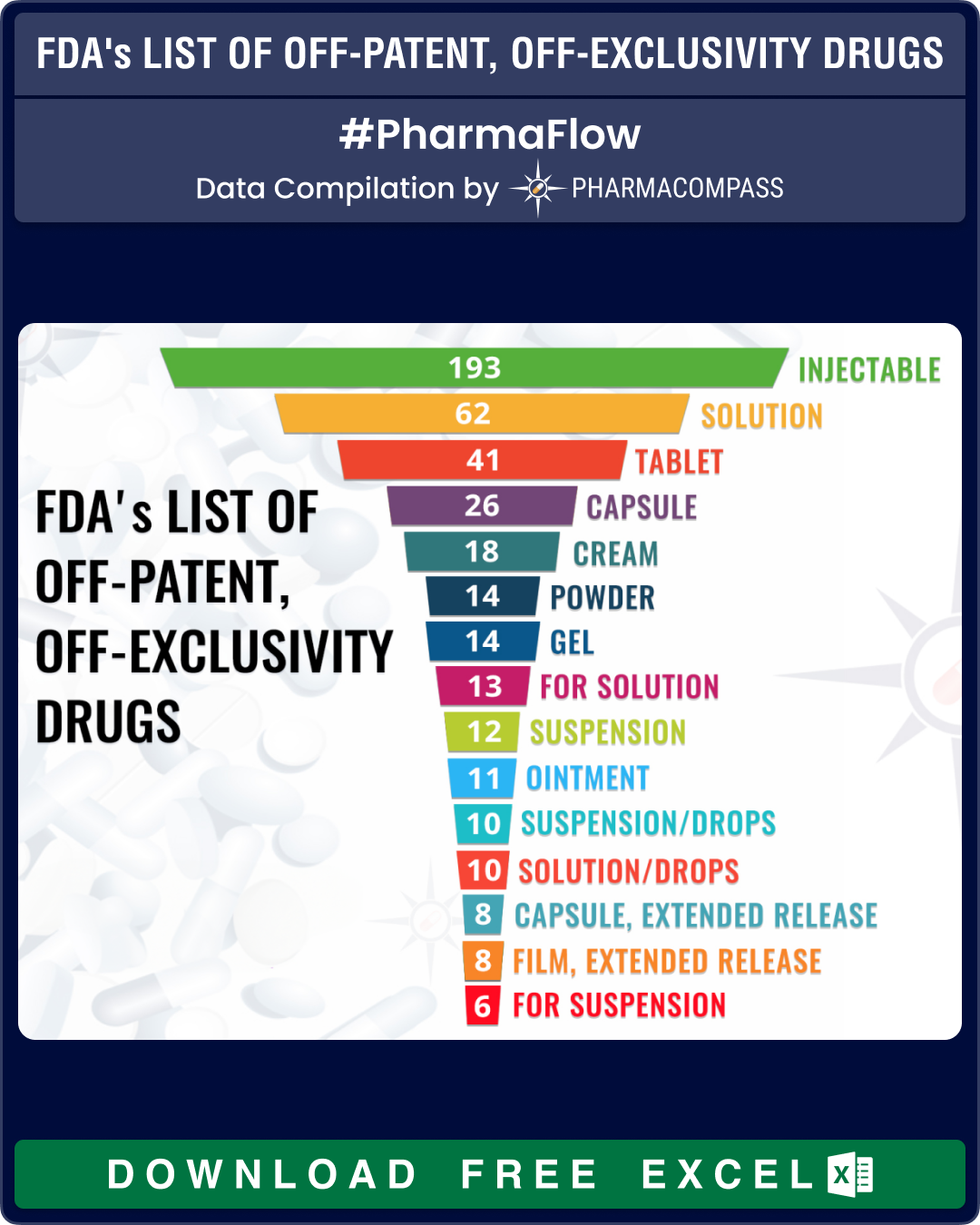
In its continuous endeavor to bolster the competitiveness of the generics market, the US Food and Drug Administration (FDA) updated its list of ‘off-patent, off-exclusivity drugs without an approved generic’. This update is part of FDA’s initiative to improve transparency and encourage the development and submission of abbreviated new drug applications (ANDAs) for drugs with limited competition.
This update follows FDA’s initiative last week to enhance one of the agency’s most viewed databases for the pharmaceutical industry — the Paragraph IV Patent Certifications List — by adding several additional data points, including the status of any 180-day exclusivity decision for individual drug products, along with other information about the dates of first approval, marketing status and expiration dates of blocking patents.
Click to get your Excel version of FDA’s List of Off-Patent, Off-Exclusivity Drugs without an Approved Generic
The agency’s intent of providing more information regarding Paragraph IV Patent Certifications is to help subsequent generic drug applicants determine when their products can be approved and marketed.
The ‘off-patent, off-exclusivity drugs without an approved generic’ includes two lists. The first list identifies drug products for which FDA could immediately accept an ANDA without prior discussion. The second list identifies drug products for which ANDA development or approval may raise potential legal, regulatory, or scientific issues that should be addressed with the FDA prior to considering submission of an ANDA.
The document also contains an appendix which identifies new drug application (NDA) drug products that were removed from the two lists because one or more ANDAs referencing, such NDA drug products, have been approved since the publication of the previous list.
Among the 11 drugs approved are the generic versions of the tablets of methylprednisolone, mifepristone, aminocaproic acid, toremifene citrate and vigabatrin and acyclovir cream.
The FDA has stated that it will update the list every six months.
China’s list for generic alternatives
The FDA isn’t the only regulator focused on increasing generic competition. As part of the Chinese government’s initiative to reform and improve the supply of generic drugs, the National Health Commission in China released a list of 34 drugs to encourage the production of their generic alternatives.
The drugs were selected from a variety used in China whose patents have expired or will soon expire, drugs that are in short supply and drugs whose manufacturers voluntarily applied for generic competition, the commission said.
The list includes commonly prescribed drugs, such as levothyroxine sodium tablets, glatiramer injection, formoterol fumarate inhalation solution, and was published on the commission’s website. The National Health Commission in China is soliciting public opinion on this list for five working days.
Much like the FDA, the National Health Commission in China will release such a list every year.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA's List of Off-Patent, Off-Exclusivity Drugs by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







