Top news of 2025: Drugmakers invest in US capacities, agree to lower Medicaid prices; Pfizer buys obesity-focused biotech Metsera
The year 2025 was an eventful one, marked by increased trade
tensions, tariff threats, accelerated
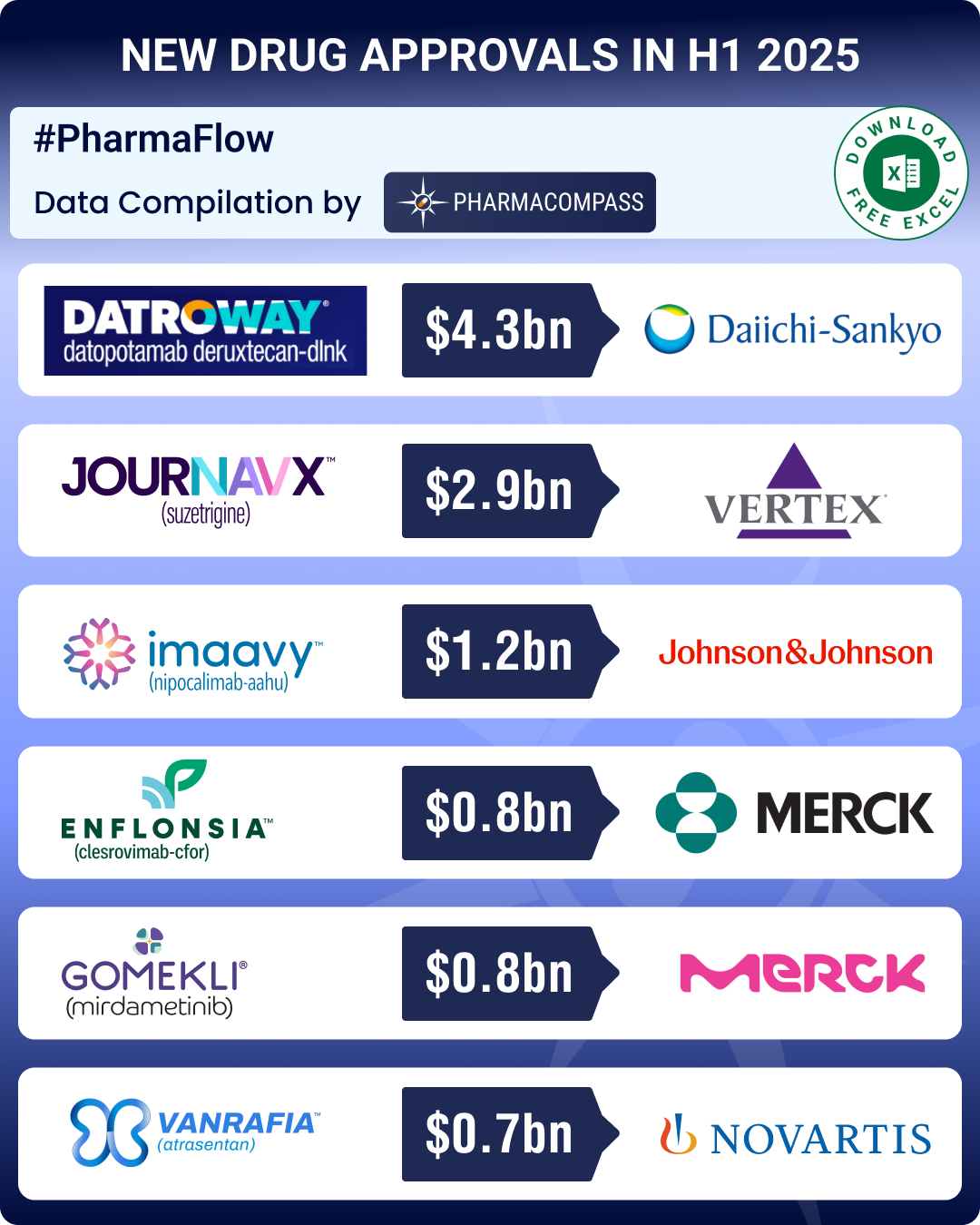
FDA approvals drop 24% in H1 2025; GSK’s UTI med, Vertex’s non-opioid painkiller lead pack of first-in-class meds
It has been a turbulent year for the US
Food and Drug Administration (FDA), marked by reduction
Top first-in-class drug candidates of 2025: Ionis’ donidalorsen, Sanofi’s fitusiran, Cytokinetics’ aficamten await FDA approval
First‑in‑class drugs are therapies with entirely new approaches that improve patient out
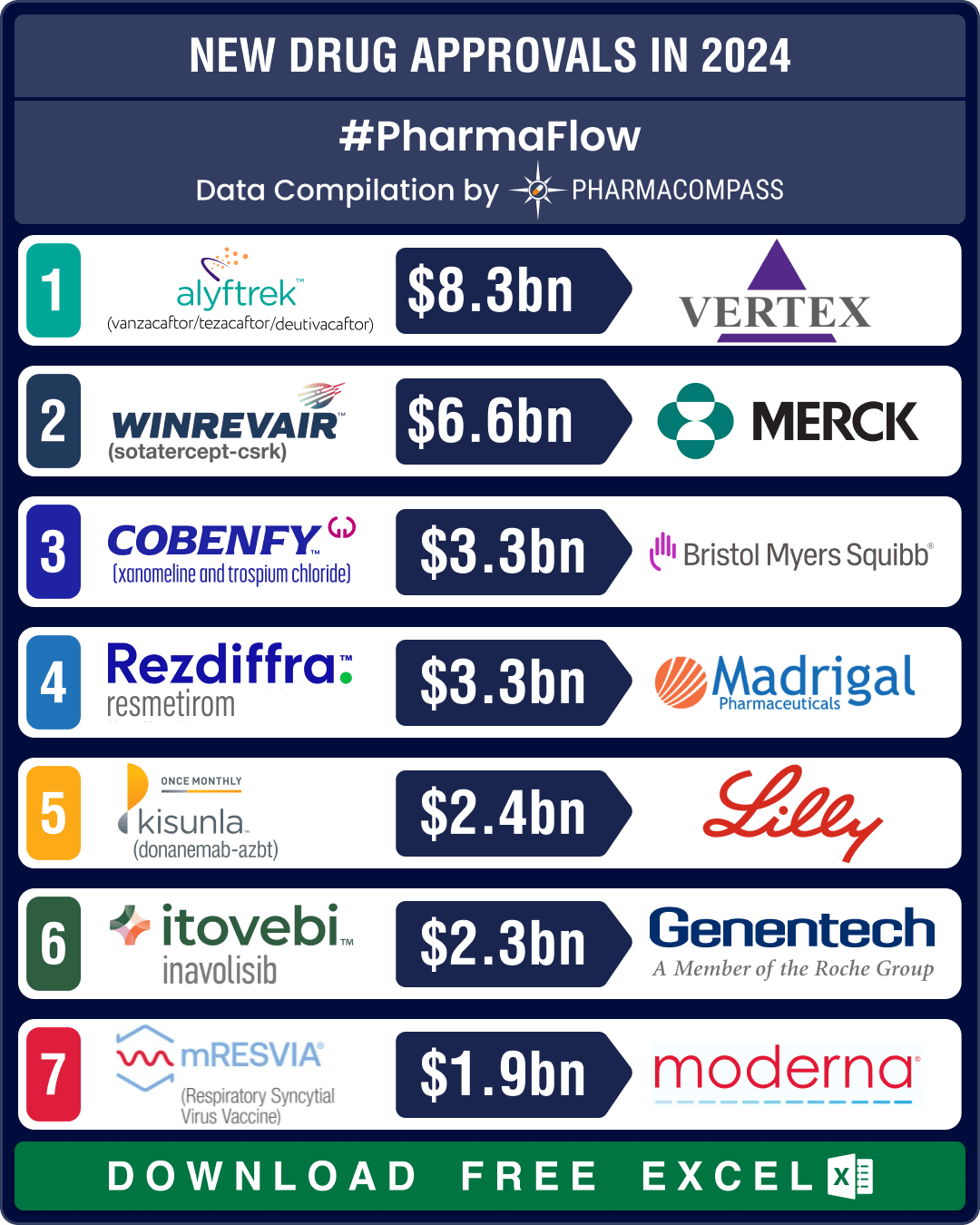
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though


 Market Place
Market Place Sourcing Support
Sourcing Support