By PharmaCompass
2019-12-19
Impressions: 5057
The development of cannabinoids in the pharmaceutical industry has exploded over the past few years. With the gradual liberation of regulations worldwide that govern cannabis and its pharmaceutical derivatives for medicinal use, this week PharmaCompass leveraged its Technology Prospector tool to develop a map of the global development landscape of these drugs.
Technology Prospector is our proprietary technology. We scanned patents and journal publications, over the past five years, for cannabinoid-related content to identify the organizations which are developing products linked to the technology of interest.
Click to view our Technology Prospector on Cannabinoids in Development
Over the past two decades, there has been a resurgence of patient interest in using cannabis and cannabinoids to treat a variety of conditions, including chronic pain, cancer pain, depression, sleep disturbances and neurological disorders. This increased interest has resulted in a renewed scientific focus on the medical use of substances found in the cannabis plant, namely cannabinoids.
The two most extensively studied ingredients in cannabis are tetrahydrocannabinol (THC) and cannabidiol (CBD). However, it is estimated that over 100 other cannabinoids and terpenoids in cannabis may also have medical uses.
THC is known to produce the psychoactive effects sought by recreational users, such as euphoria, relaxation and heightened sensory experiences. There is also evidence to support the medical use of THC in controlling nausea and vomiting, stimulating appetite and reducing pain. CBD, on the other hand, may moderate the psychoactive effects of THC, while also reducing epileptic seizures.
In the early 1990s, a cannabinoid system in the human brain and body was discovered that was responsible for the control of important biological functions, such as cognition, memory, pain, sleep and immune functioning.
In view of this increased interest in cannabinoids, we are leveraging PharmaCompass’ Technology Prospector to provide an overview of the organizations actively involved in developing products linked to cannabinoids.
Click to view our Technology Prospector on Cannabinoids in Development
Cannabinoid
products on market
Several cannabinoid-containing medicinal products have been on the market for many years. Dronabinol (brand name: Marinol), a synthetic form of THC, got approved in 1985 for prevention of nausea and vomiting during chemotherapy and hauled in over US$ 100 million in annual sales. After the initial launch, the product also got approval for appetite and weight loss in patients with HIV/AIDS. In 1998, the product got reclassified from a Schedule II drug to a Schedule III drug by the Drug Enforcement Administration (DEA) in the US.
In order to prevent drug abuse, the DEA has divided these substances into five categories (or Schedules) based on each drug’s (1) potential for abuse, (2) safety, (3) addictive potential and (4) whether or not it has any legitimate medical applications. The potential for drug abuse is the highest for Schedule I drugs and lowest for Schedule V drugs.
Nabilone (brand names: Cesamet and Canemes) is a synthetic cannabinoid similar to THC. The main indication for its use is nausea and vomiting associated with chemotherapy, usually after previous treatments have failed.
GW Pharmaceuticals’ Sativex, a mixture of equal quantities of THC and CBD from two cannabis extracts, is approved for sales in countries for the treatment of spasticity (muscle spasms and stiffness) related to multiple sclerosis — a disease that affects 1.3 million people worldwide, of which up to 80 percent suffer from spasticity.
In 2018, the US Food and Drug Administration (FDA) approved GW Pharmaceuticals’ Epidiolex (cannabidiol or CBD) oral solution for the treatment of seizures associated with two rare and severe forms of epilepsy — Lennox-Gastaut syndrome and Dravet syndrome.
The drug can be taken by patients aged two years and above.
Click to view our Technology Prospector on Cannabinoids in Development
The battle of
synthetic versus natural Cannabinoids
In order to win approval for Epidiolex, GW Pharmaceuticals set up giant greenhouses in the United Kingdom where it raises cannabis plants under highly controlled conditions. The plants are then made to undergo extraction and purification to crystallize pure CBD at GW’s facilities.
There are other firms which are supporting production of cannabinoids via organic synthesis rather than extraction from the cannabis plant. There are already multiple Drug Master Files (DMF) submissions to the FDA for synthetic cannabidiol, as companies bet that the future of cannabinoid production will be from organic chemistry.
Firms like Noramco and Johnson Matthey, which have conventionally been active in producing controlled substances from opium like morphine, codeine etc, have focused significant resources to meet the increasing demand for large-scale production of cannabinoids. While there are multiple technical challenges associated with scaling up organic synthesis processes, once overcome, they can usually meet growing market needs faster than processes dependent on plant extraction.
However, GW has claimed the superiority of cannabinoid extracts over synthesized molecules. According to GW, cannabinoid ratios in the plant generate a complex chemical fingerprint which plays a critical role in therapeutic efficacy.
While Epidiolex is more than 98 percent CBD, GW’s first product — Sativex — is a mixture of CBD and THC. However, to get regulatory approvals for synthetic cannabinoids maybe easier, since they have a well characterized starting material and a more precisely defined pathway that leads to more consistent manufacturing.
Click to view our Technology Prospector on Cannabinoids in Development
New developments
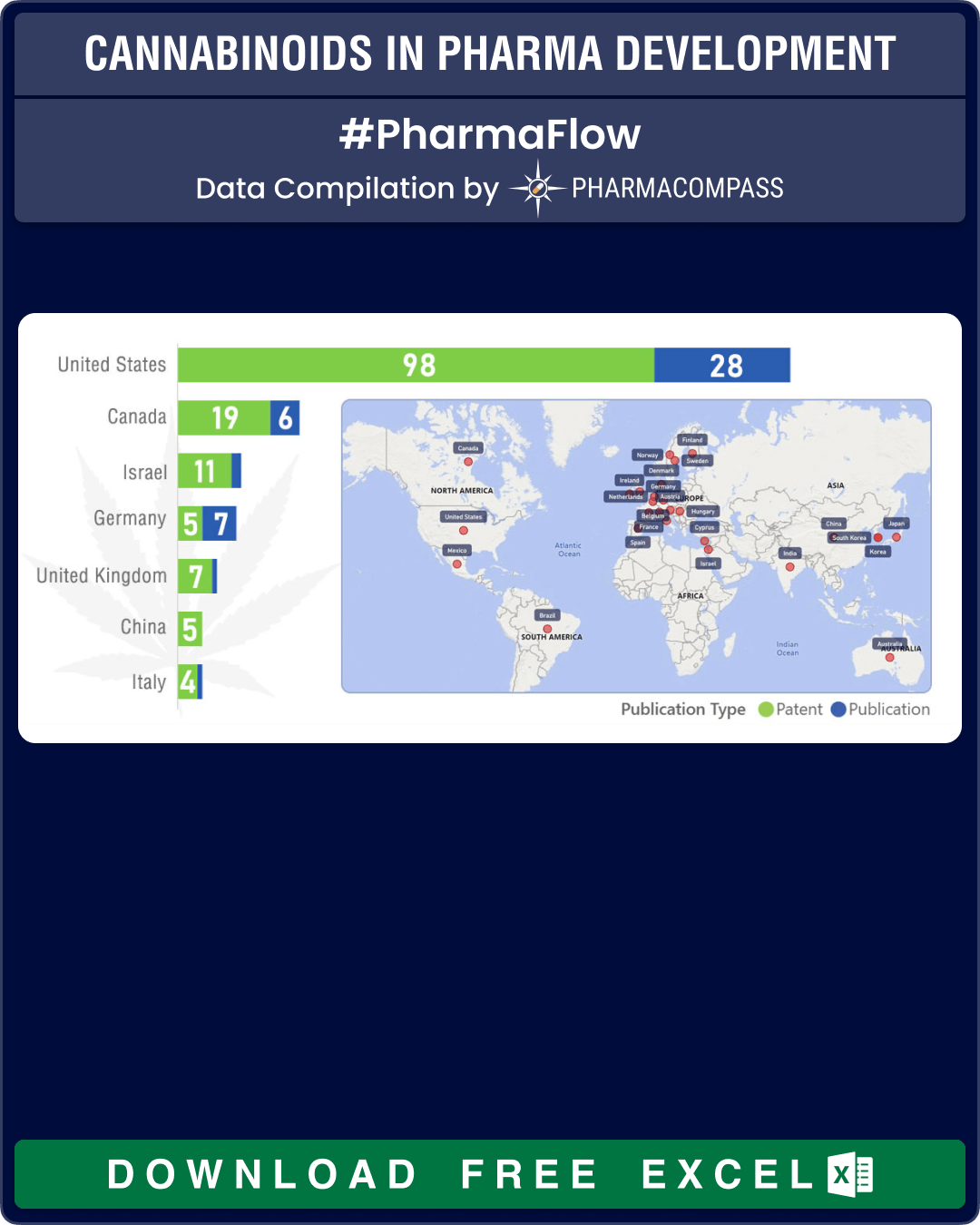
As the developments on cannabinoids evolve rapidly, PharmaCompass’ Technology Prospector found over 200 organizations across 25 countries that had either patented or published work related to cannabinoids. The United States has the maximum active organizations followed by Canada, Israel and then Germany.
Unsurprisingly, GW Pharmaceutical leads the count of patents filed. However, many major pharmaceutical companies such as Roche, AbbVie, AstraZeneca and Merck are developing products targeting the cannabinoid system in the human brain and body.
To deliver cannabinoids, while companies like US-based Axim Biotechnologies are working on delivering cannabinoids via chewing gums to treat nausea, Hanyi Biological in China has patents on the delivery of cannabinoids as a toothpaste and mouthwash to treat irritable bowel syndrome.
Botanix Pharmaceuticals is working on new treatment options for acne, psoriasis and atopic dermatitis, using a transdermal drug delivery system.
There are also new manufacturing technologies being developed at companies like US-based Teewinot Life Sciences that have developed processes to manufacture cannabinoids using biocatalysis and established players like Noramco have signed an agreement with them.
Click to view our Technology Prospector on Cannabinoids in Development
Our view
As government controls ease on the use of cannabis for scientific and medical purposes and there is a growing understanding that CBD should not be placed under international drug control because the substance does not have psychoactive properties, with no reported cases of abuse or dependence, there is clear evidence that the field will witness a lot more development activity in the years to come.
PharmaCompass’ Technology Prospector, our proprietary technology to identify organizations which are developing products linked to the technology of interest, can be applied across a variety of capabilities. Email us at support@pharmacompass.com to learn more.
Click to view our Technology Prospector on Cannabinoids in Development
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Cannabinoids in Pharma Development by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







