



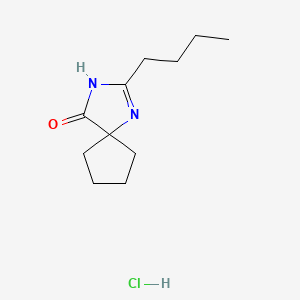

1. 151257-01-1
2. 2-n-butyl-1,3-diaza-spiro[4,4]non-1-en-4-one Hydrochloride
3. 2-butyl-1,3-diaza-spiro[4.4]non-1-en-4-one Hcl
4. 2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one;hydrochloride
5. 1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-, Monohydrochloride
6. Mfcd03428357
7. 2-butyl-1,3-diazaspiro(4.4)non-1-en-4-one Hydrochloride
8. 2-butyl-4-spirocyclopentane-2-imidazolin-5-one, Hcl
9. 1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-, Hydrochloride (1:1)
10. 2-butyl-4-spirocyclopentane-2-imidazolin-5-one Hydrochloride
11. 2-n-butyl-1,3-diaza-spiro[4,4]non-1-en-4-one Hcl
12. Ec 424-560-4
13. Schembl535435
14. 2-butyl-1,3-diazaspiro[4.4]non-1-en-4-onehydrochloride
15. Dtxsid10436477
16. 2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one--hydrogen Chloride (1/1)
17. Akos015907796
18. Akos037643283
19. Ab15412
20. Ac-6864
21. Am90294
22. Cs-w014181
23. As-12497
24. Sy036928
25. B3291
26. Ft-0659370
27. 257b011
28. A809140
29. 2-butyl-1,3-diazaspiro[4,4]non-1-en-4-one Hcl
30. 2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one Hcl
31. 2n-butyl-1,3-diazaspiro (4,4) Non-1-en-4 One Hcl
32. 2n-butyl-1,3-diazaspiro (4,4) Non-1-en-4-one Hcl
33. 2-butyl-1,3-diaza-spiro[4.4]non-1-en-4-one Hydrochloride
34. 2-butyl-1,3-diaza-spiro[4.4]non-1-en4-one Monohydrochloride
35. 2-butyl-1,3-diazaspiro[4,4]non-1-en-4-one Monohydrochloride
36. 2-butyl-1,3-diazaspiro[4.4]non-2-en-4-one Monohydrochloride
37. 2-(n-butyl)-1,3-diazaspiro[4.4]non-1-ene-4-one Hydrochloride
38. 2-butyl-1,3-diaza-spiro [4.4] Non-1-en-4-one Monohydrochloride
39. 2-n-butyl-1,3-diaza-spiro[4,4]non-1-en-4-onehydrochloride
40. 8-butyl-7,9-diazaspiro[4.4]non-8-en-6-one Hydrochloride;2-n-butyl-1,3-diaza-spiro[4,4]non-1-en-4-one Hcl
| Molecular Weight | 230.73 g/mol |
|---|---|
| Molecular Formula | C11H19ClN2O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 230.1185909 g/mol |
| Monoisotopic Mass | 230.1185909 g/mol |
| Topological Polar Surface Area | 41.5 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 265 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |







