

1. Allohexal
2. Allohexan
3. Alloprin
4. Allopurin
5. Allorin
6. Allpargin
7. Allural
8. Apulonga
9. Apurin
10. Atisuril
11. Bleminol
12. Caplenal
13. Capurate
14. Cellidrin
15. Embarin
16. Foligan
17. Hamarin
18. Jenapurinol
19. Lopurin
20. Lysuron
21. Milurit
22. Milurite
23. Novopurol
24. Pan Quimica
25. Progout
26. Pureduct
27. Purinol
28. Remid
29. Rimapurinol
30. Roucol
31. Suspendol
32. Tipuric
33. Uribenz
34. Uridocid
35. Uripurinol
36. Urosin
37. Urtias
38. Xanthomax
39. Xanturic
40. Zygout
41. Zyloprim
42. Zyloric
1. 315-30-0
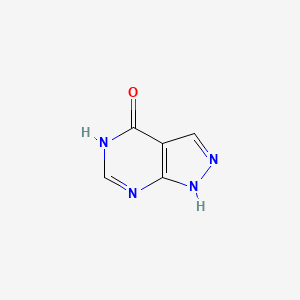
2. 1h-pyrazolo[3,4-d]pyrimidin-4-ol
3. 4-hydroxypyrazolo[3,4-d]pyrimidine
4. Allopurinolum
5. 4-hpp
6. Alopurinol
7. 1h-pyrazolo(3,4-d)pyrimidin-4-ol
8. 4-hydroxypyrazolopyrimidine
9. 4-hydroxy-1h-pyrazolo(3,4-d)pyrimidine
10. 4-hydroxy-3,4-pyrazolopyrimidine
11. 4-hydroxypyrazolo(3,4-d)pyrimidine
12. 1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-one
13. Sigapurol
14. Uritas
15. 4-hydroxypyrazolyl(3,4-d)pyrimidine
16. 4h-pyrazolo(3,4-d)pyrimidin-4-one
17. 4'-hydroxypyrazolol(3,4-d)pyrimidine
18. Bw 56-158
19. Al-100
20. 1,5-dihydro-4h-pyrazolo(3,4-d)pyrimidin-4-one
21. Bw-56-158
22. 1,5-dihydro-4h-pyrazolo(3,4-d)pyrimidine-4-one
23. Chebi:40279
24. 63cz7gjn5i
25. Nsc-101655
26. Dtxsid4022573
27. Nsc1390
28. Nsc101655
29. 4-hydroxy-1h-pyrazolo[3,4-d]pyrimidine
30. Dtxcid502573
31. Duzallo Component Allopurinol
32. Allopurinol (mart.)
33. Allopurinol [mart.]
34. Allopurinol (usp-rs)
35. Allopurinol [usp-rs]
36. Allopurinol (ep Impurity)
37. Allopurinol [ep Impurity]
38. Allopurinol (ep Monograph)
39. Allopurinol [ep Monograph]
40. Allopurinol (usp Monograph)
41. Allopurinol [usp Monograph]
42. 4-hydroxypyrazolyl[3,4-d]pyrimidine
43. 4'-hydroxypyrazolol[3,4-d]pyrimidine
44. Allopurinolo
45. Zurinol
46. 4'-hpp
47. M04aa01
48. 1h-pyrazolo(3,4-d)pyrimdin-4-ol
49. 1,5-dihydro-4h-pyrazolo(3,4-d)pryimidin-4-one
50. 206-250-9
51. Zyloprim
52. Lopurin
53. Zyloric
54. Suspendol
55. Atisuril
56. Bleminol
57. Caplenal
58. Takanarumin
59. Uripurinol
60. Embarin
61. Foligan
62. Milurit
63. Progout
64. Urosin
65. Anoprolin
66. Cellidrin
67. Epidropal
68. Ailural
69. Allopur
70. Allural
71. Alositol
72. Bloxanth
73. Cosuric
74. Hamarin
75. Ledopur
76. Lysuron
77. Uricemil
78. Uriprim
79. Xanturat
80. Aloral
81. Anzief
82. Apurin
83. Apurol
84. Geapur
85. Gotax
86. Remid
87. Urbol
88. Urolit
89. Urtias
90. Ketobun-a
91. Apulonga
92. Dabrosin
93. Dabroson
94. Ketanrift
95. Miniplanor
96. Nektrohan
97. Adenock
98. Allozym
99. Aluline
100. Gichtex
101. Monarch
102. Riball
103. 1h-pyrazolo[3,4-d]pyrimidin-4(5h)-one
104. Hexanuret
105. Epuric
106. Allo-puren
107. Allopurinol(i)
108. Dura Al
109. 180749-08-0
110. 73334-58-4
111. 180749-06-8
112. Urtias 100
113. 1h-pyrazolo[3,4-d]pyrimidin-4(7h)-one
114. 2h-pyrazolo[3,4-d]pyrimidin-4-ol
115. Nsc-1390
116. Alopurinol [inn-spanish]
117. Allopurinolum [inn-latin]
118. 1h-pyrazolo[3,4-d]pyrimidin-4(2h)-one
119. 180749-09-1
120. Zyloprim (tn)
121. 916980-04-6
122. Mfcd00599413
123. 1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one
124. 9002-17-9
125. B. W. 56-158
126. 4h-pyrazolo(3,4-d)pyrimidin-4-one, 1,5-dihydro-
127. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 1,5-dihydro-
128. 180749-07-9
129. 184789-03-5
130. 1h,4h,7h-pyrazolo[3,4-d]pyrimidin-4-one
131. Mls000069453
132. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 1,2-dihydro-
133. 1h,2h,4h-pyrazolo[3,4-d]pyrimidin-4-one
134. Xanthine Oxidase
135. Smr000059083
136. 1h-pyrazolo[3,4-d]pyrimidin-4-ol (9ci)
137. 291279-53-3
138. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 1,7-dihydro- (9ci)
139. Ncgc00015094-02
140. Ncgc00094580-04
141. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 2,5-dihydro- (9ci)
142. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 2,7-dihydro- (9ci)
143. Bw-56158
144. 1h,4h,5h-pyrazolo[3,4-d]pyrimidin-4-one
145. 1,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one
146. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 1,7-dihydro-
147. 4h-pyrazolo[3, 1,5-dihydro-
148. Ailurial
149. Wln: T56 Bmn Gn Inj Fq
150. Nsc 1390
151. Cas-315-30-0
152. Ccris 626
153. Nsc 101655
154. Hsdb 3004
155. Sr-05000001983
156. Einecs 206-250-9
157. Unii-63cz7gjn5i
158. Uricto
159. Ath008
160. 4-hydroxypyrazol[3,4-d]pyrimidine
161. Prestwick_511
162. Xanthomax-100
163. Xanthomax-300
164. Aluline 100
165. Aluline 300
166. Hamarin 100
167. Hamarin 300
168. Zyloric-300
169. Allopurinol [usan:usp:inn:ban:jan]
170. Allopurinol (standard)
171. Allopurinol (zyloprim)
172. Spectrum_000026
173. Allopurinol - Ep Grade
174. Allopurinol [mi]
175. Opera_id_1680
176. Spectrum2_000098
177. Spectrum3_000289
178. Spectrum4_000135
179. Spectrum5_000768
180. Allopurinol [inn]
181. Allopurinol [jan]
182. Lopac-a-8003
183. 1,4-d]pyrimidin-4-one
184. Allopurinol [hsdb]
185. Allopurinol [usan]
186. A 8003
187. Cid_2094
188. Schembl4627
189. A1bd4
190. Chembl1467
191. Nciopen2_001825
192. Lopac0_000102
193. Allopurinol [who-dd]
194. Allopurinol [who-ip]
195. Bspbio_001798
196. Kbiogr_000550
197. Kbioss_000386
198. Mls001148183
199. Us9138393, Allopurinol
200. Us9144538, Allopurinol
201. Divk1c_000685
202. Spectrum1500108
203. Spbio_000056
204. Allopurinolum [who-ip]
205. Gtpl6795
206. Schembl1128219
207. Allopurinol (jp18/usp/inn)
208. Bdbm35440
209. Hms502c07
210. Hy-b0219r
211. Kbio1_000685
212. Kbio2_000386
213. Kbio2_002954
214. Kbio2_005522
215. Kbio3_001298
216. Allopurinol [orange Book]
217. Ninds_000685
218. Bdbm181133
219. Hms1920a15
220. Hms2091g15
221. Hms2234m09
222. Hms3259k13
223. Hms3260e06
224. Hms3371i11
225. Hms3651o13
226. Hms3714l22
227. Pharmakon1600-01500108
228. Bcp26973
229. Hy-b0219
230. Str05189
231. Tox21_110082
232. Tox21_200922
233. Tox21_500102
234. 4-hydroxy-pyrazolo[3,4-d]pyrimidin
235. Ac-019
236. Bbl009959
237. Bdbm50016784
238. Bdbm50140241
239. Ccg-38916
240. Nsc755858
241. S1630
242. Sc1118
243. Sc2251
244. Stk378584
245. Stk711106
246. Akos000267490
247. Akos000269759
248. Akos024255717
249. Tox21_110082_1
250. Allopurinol, Xanthine Oxidase Inhibitor
251. Ccg-204197
252. Ccg-221406
253. Ccg-266128
254. Db00437
255. Fa17288
256. Lp00102
257. Nc00492
258. Nsc-755858
259. Sb10164
260. Sdccgsbi-0050090.p005
261. Idi1_000685
262. Ncgc00015094-01
263. Ncgc00015094-03
264. Ncgc00015094-04
265. Ncgc00015094-05
266. Ncgc00015094-06
267. Ncgc00015094-07
268. Ncgc00015094-08
269. Ncgc00015094-18
270. Ncgc00015094-22
271. Ncgc00091134-01
272. Ncgc00091134-02
273. Ncgc00091134-03
274. Ncgc00094580-01
275. Ncgc00094580-02
276. Ncgc00094580-05
277. Ncgc00188948-01
278. Ncgc00258476-01
279. Ncgc00260787-01
280. Fa160361
281. Fa162226
282. Ts-00028
283. Sbi-0050090.p004
284. Db-065332
285. Db-261136
286. Db-272461
287. Db-272465
288. Db-272466
289. Db-272469
290. 2h-pyrazolo[3,4-d]pyrimidin-4-ol (9ci)
291. A0907
292. Eu-0100102
293. Ns00000618
294. Sw199406-4
295. 1,5-dihydropyrazolo[3,4-d]-pyrimidin-4-one
296. En300-34144
297. Vu0611037-1
298. Bim-0061756.0001
299. D00224
300. F18007
301. Ab00173448-03
302. Ab00173448-04
303. Ab00173448_05
304. Ab01274719-01
305. Ab01274719_02
306. Ab-323/25048497
307. Allopurinol (4-hydroxypyrazolo[3,4-d]pyrimidine)
308. Q412486
309. Sr-01000075595
310. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 2,5-dihydro-
311. Sr-01000075595-1
312. Sr-05000001983-1
313. Sr-05000001983-2
314. Brd-k86307448-001-16-7
315. Brd-k86307448-001-17-5
316. Brd-k86307448-001-19-1
317. F2173-0394
318. F3329-0375
319. Z104486670
320. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 1,2-dihydro- (9ci)
321. 4h-pyrazolo[3,4-d]pyrimidin-4-one, 2,7-dihydro-
322. Allopurinol, British Pharmacopoeia (bp) Reference Standard
323. Allopurinol, European Pharmacopoeia (ep) Reference Standard
324. Allopurinol, United States Pharmacopeia (usp) Reference Standard
325. 1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-one Synonym: Allopurinol
326. Allopurinol, Pharmaceutical Secondary Standard; Certified Reference Material
327. Inchi=1/c5h4n4o/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2h,(h2,6,7,8,9,10
328. 1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-one;4-hydroxypyrazolo[3,4-d]pyrimidine;4-oxopyrazolo[3,4-d]pyrimidine
329. 1,5dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-one;4-hydroxypyrazolo[3,4-d]pyrimidine;4-oxopyrazolo[3,4-d]pyrimidine
| Molecular Weight | 136.11 g/mol |
|---|---|
| Molecular Formula | C5H4N4O |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 70.1 |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 190 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antimetabolites; Antimetabolites, Antineoplastic; Enzyme Inhibitors; Gout Suppressants
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Allopurinol is indicated in the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Allopurinol (allopurinol) tablet (April 2007). Available from, as of February 24, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3795
Allopurinol is indicated in the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for over production of uric acid is no longer present. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Allopurinol (allopurinol) tablet (April 2007). Available from, as of February 24, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3795
Allopurinol is indicated in the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Allopurinol (allopurinol) tablet (April 2007). Available from, as of February 24, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3795
For more Therapeutic Uses (Complete) data for Allopurinol (9 total), please visit the HSDB record page.
Since allopurinol and oxypurinol are distributed into milk, allopurinol should be used with caution in nursing women.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3690
Results of early clinical studies and experience suggested that some allopurinol-induced adverse effects (eg, acute attacks of gout, rash) occurred in more than 1% of patients, but current experience suggests that adverse effects of the drug occur in less than 1% of patients. The reduced incidence in adverse effects observed with more recent experience may have resulted in part from initiating therapy with the drug more gradually and following current prescribing precautions and recommendations.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3689
The most common adverse effect of oral allopurinol is a pruritic maculopapular rash. Dermatitides of the exfoliative, urticarial, erythematous, eczematoid, hemorrhagic, and purpuric types have also occurred. Alopecia, fever, and malaise may also occur alone or in conjunction with dermatitis. In addition, severe furunculoses of the nose, cellulitis, and ichthyosis have been reported. The incidence of rash may be increased in patients with renal insufficiency. Skin reactions may be delayed and have been reported to occur as long as 2 years after initiating allopurinol therapy. Rarely, skin rash may be followed by severe hypersensitivity reactions which may sometimes be fatal. Some patients who have developed severe dermatitis have also developed cataracts (including a case of toxic cataracts), but the exact relationship between allopurinol and cataracts has not been established. Pruritus, onycholysis, and lichen planus have also occurred rarely in patients receiving allopurinol. Facial edema, sweating, and skin edema have also occurred rarely, but a causal relationship to the drug has not been established.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3689
Local injection site reactions have been reported in patients receiving allopurinol sodium iv.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3689
For more Drug Warnings (Complete) data for Allopurinol (28 total), please visit the HSDB record page.
Allopurinol is indicated in: 1) the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy). 2) the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for overproduction of uric acid is no longer present. 3) the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks.
FDA Label
Allopurinol, a xanthine oxidase inhibitor, is a urate-lowering medication.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
M04AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M04 - Antigout preparations
M04A - Antigout preparations
M04AA - Preparations inhibiting uric acid production
M04AA01 - Allopurinol
M04AA01
Absorption
This drug is about 90% absorbed from the gastrointestinal tract. Peak plasma levels normally occur at 1.5 hours and 4.5 hours post-dose for allopurinol and oxipurinol respectively. Following one oral dose of 300 mg of allopurinol, maximum plasma levels of about 3 mcg/mL of allopurinol and 6.5 mcg/mL of oxipurinol were measured.
Route of Elimination
Approximately 80% of orally ingested allopurinol is found excreted in the urine as various metabolites. About 20% of ingested allopurinol is excreted in the feces.
Volume of Distribution
Allopurinol and oxypurinol are both substrates for the enzyme xanthine oxidase, which is present in the cytoplasm of endothelial cells of capillaries, including sinusoids, with the highest activity demonstrated in the liver and intestinal lining. Tissue concentrations of allopurinol have not yet been reported in humans, however, it is probable that allopurinol and the metabolite oxypurinol would be measured in the highest concentrations in the abovementioned tissues. In animals, allopurinol concentrations are found to reach the highest levels in the blood, liver, intestine and heart, and lowest in the brain and lung tissues.
Clearance
Since allopurinol and its metabolites are mainly eliminated by the kidney, accumulation of this drug can occur in patients with renal dysfunction or failure, and the dose of allopurinol should, therefore, be reduced. With a creatinine clearance of 10 to 20 mL/min, a daily dosage of 200 mg of allopurinol is suitable. When the creatinine clearance is less than 10 mL/min, the daily dosage should not be higher than 100 mg. With severe renal impairment (creatinine clearance measured at less than 3 mL/min) a longer interval between doses may be required.
Following oral administration, approximately 80-90% of a dose of allopurinol is absorbed from the GI tract. Peak plasma concentrations of allopurinol are reached 2-6 hours after a usual dose.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Allopurinol is absorbed poorly following rectal administration of the drug as suppositories (in a cocoa butter or polyethylene glycol base). Plasma allopurinol or oxipurinol concentrations have been minimal or undetectable following such rectal administration.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Following oral administration of single 100- or 300-mg dose of allopurinol in healthy adult males in one study, peak plasma allopurinol concentrations of about 0.5 or 1.4 ug/mL, respectively, occurred in about 1-2 hours, while peak oxypurinol (the active metabolite of allopurinol) concentrations of about 2.4 and 6.4 ug/mL, respectively, were reached within about 3-4 hours. In the same study, following iv infusion over 30 minutes of a single 100- or 300-mg dose of allopurinol (as allopurinol sodium), peak plasma concentrations of about 1.6 and 5.1 ug/mL, respectively, occurred in about 30 minutes, while peak oxypurinol concentrations of about 2.2 and 6.2 ug/mL, respectively, were reached within about 4 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Following intravenous administration in six healthy male and female subjects, allopurinol was rapidly eliminated from the systemic circulation primarily via oxidative metabolism to oxypurinol, with no detectable plasma concentration of allopurinol after 5 hours post dosing. Approximately 12% of the allopurinol intravenous dose was excreted unchanged, 76% excreted as oxypurinol, and the remaining dose excreted as riboside conjugates in the urine. The rapid conversion of allopurinol to oxypurinol was not significantly different after repeated allopurinol dosing. ... Oxypurinol was primarily eliminated unchanged in urine by glomerular filtration and tubular reabsorption, with a net renal clearance of about 30 mL/min.
US Natl Inst Health; DailyMed. Current Medication Information for Allopurinol (allopurinol) tablet (April 2007). Available from, as of February 24, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3795
For more Absorption, Distribution and Excretion (Complete) data for Allopurinol (13 total), please visit the HSDB record page.
Allopurinol is rapidly metabolized to the corresponding xanthine analog, oxipurinol (alloxanthine), which is also an inhibitor of xanthine oxidase enzyme. Both allopurinol and oxypurinol inhibit the action of this enzyme. Allopurinol and oxypurinol are also converted by the purine salvage pathway to their respective ribonucleotides. The effect of these ribonucleotides related to the hypouricemic action of allopurinol in humans is not fully elucidated to this date. These metabolites may act to inhibit de novo purine biosynthesis by inhibiting the enzyme, _amidophosphoribosyltransferase_. The ribonucleotides have not been found to be incorporated in DNA.
Allopurinol and allopurinol sodium are rapidly metabolized by xanthine oxidase to oxypurinol, which is pharmacologically active. Rapid metabolism of allopurinol to oxypurinol does not seem to be affected substantially during multiple dosing. Pharmacokinetic parameters (eg, AUC, plasma elimination half-lives) of oxypurinol appear to be similar following oral administration of allopurinol and iv administration of allopurinol sodium.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Both allopurinol and oxypurinol are conjugated and form their respective ribonucleosides.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Allopurinol-1-riboside, a major metabolite of allopurinol, is commonly thought to be directly synthesized by purine nucleoside phosphorylase (PNP) in vivo. As this enzyme is otherwise believed to function in vivo primarily in the direction of nucleoside breakdown, we have determined by high performance liquid chromatography and a conventional chromatographic method the urinary metabolites of allopurinol in a child deficient of PNP. In this patient approximately 40% of urinary allopurinol metabolites consisted of allopurinol-1-riboside, thus proving the possibility of indirect formation of allopurinol-1-riboside via allopurinol-1-ribotide in vivo, catalysed by hypoxanthine guanine phosphoribosyltransferase (HGPRT) and a phosphatase.
PMID:6409116 Reiter S et al; Biochem Pharmacol 32 (14): 2167-74 (1983).
... The major and active metabolite, oxypurinol, is detected in the circulation within 15 minutes of allopurinol administration. Oxypurinol concentrations are higher than those of the parent drug and accumulation occurs during long term administration. ...Oxypurinol is eliminated by the kidney and has a much longer elimination half-life than allopurinol. Oxypurinol accumulates in patients with renal dysfunction; hence allopurinol dosages should be adjusted in such patients. ...
PMID:3536254 Murrell GA, Rapeport WG; Clin Pharmacokinet 11 (5): 343-53 (1986).
For more Metabolism/Metabolites (Complete) data for Allopurinol (7 total), please visit the HSDB record page.
Hepatic Route of Elimination: Approximately 20% of the ingested allopurinol is excreted in the feces. Half Life: 1-3 hours
The plasma half-life of allopurinol is 1-2 hours, due to its rapid renal clearance.
The half-lives of allopurinol and oxypurinol are about 1-3 hours and 18-30 hours, respectively, in patients with normal renal function and are increased in patients with renal impairment.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Allopurinol is rapidly cleared from plasma with half-time of 2-3 hr, primarily by conversion to alloxanthine.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 677
Serum half-life of allopurinol is 39 min.
PMID:639435 Hande K et al; Clin Pharmacol Ther23 (5): 598-605 (1978)
Allopurinol is a structural analog of the natural purine base, hypoxanthine. After ingestion, allopurinol is metabolized to its active metabolite, oxypurinol (_alloxanthine_) in the liver, which acts as an inhibitor of xanthine oxidase enzyme. Allopurinol and its active metabolite inhibit xanthine oxidase, the enzyme that converts hypoxanthine to xanthine and xanthine to uric acid. Inhibition of this enzyme is responsible for the effects of allopurinol. This drug increases the reutilization of hypoxanthine and xanthine for nucleotide and nucleic acid synthesis by a process that involves the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase). This process results in an increased nucleotide concentration, which causes feedback inhibition of de novo purine synthesis. The end result is decreased urine and serum uric acid concentrations, which decreases the incidence of gout symptoms. Accompanying the reduction of serum uric acid by allopurinol is an increase in the serum and urine concentrations of hypoxanthine and xanthine (due to inhibition of xanthine oxidase). In the absence of allopurinol, regular urinary excretion of oxypurines almost entirely occurs in the form of uric acid. After the ingestion of allopurinol, the contents of excreted urine are hypoxanthine, xanthine, and uric acid. Because each substance has its own individual solubility, the concentration of uric acid in plasma is decreased without exposing the renal tissues to a high load of uric acid, thereby decreasing the risk of crystalluria. By lowering the uric acid concentration in the plasma below its limits of solubility, allopurinol encourages the dissolution of gout tophi. Although the levels of hypoxanthine and xanthine are found to be increased after allopurinol ingestion, the risk of deposition in renal tissues is less than that of uric acid, as they become more soluble and are rapidly excreted by the kidney.
Allopurinol inhibits xanthine oxidase, the enzyme that catalyzes the conversion of hypoxanthine to xanthine and of xanthine to uric acid. Oxypurinol, a metabolite of allopurinol, also inhibits xanthine oxidase. By inhibiting xanthine oxidase, allopurinol and its metabolite block conversion of the oxypurines (hypoxanthine and xanthine) to uric acid, thus decreasing serum and urine concentrations of uric acid. The drug differs, therefore, from uricosuric agents which lower serum urate concentrations by promoting urinary excretion of uric acid. Xanthine oxidase concentrations are not altered by long-term administration of the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3690
Allopurinol does not directly interfere with purine nucleotide or nucleic acid synthesis. The drug, however, indirectly increases oxypurine and allopurinol ribonucleotide concentrations and decreases phosphoribosylpyrophosphate concentrations, thus decreasing de novo purine biosynthesis by pseudofeedback inhibition. In addition, allopurinol increases the incorporation of hypoxanthine and xanthine into DNA and RNA, thereby further decreasing serum urate concentrations. Allopurinol may produce a deficit of total purines (uric acid and oxypurines) amounting to several hundred mg daily.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3690
Accompanying the decrease in uric acid produced by allopurinol is an increase in serum and urine concentrations of hypoxanthine and xanthine. Plasma concentrations of these oxypurines do not, however, rise commensurately with the fall in serum urate concentrations and are often 20-30% less than would be expected in view of urate concentrations prior to allopurinol therapy. This discrepancy occurs because renal clearance of the oxypurines is at least 10 times greater than that of uric acid. In addition, normal urinary purine output is almost exclusively uric acid, but after treatment with allopurinol, it is composed of uric acid, xanthine, and hypoxanthine, each having independent solubility. Thus, the risk of crystalluria is reduced. Alkalinization of the urine increases the solubility of the purines, further minimizing the risk of crystalluria. Decreased tubular transport of uric acid also results in increased renal reabsorption of calcium and decreased calcium excretion.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Allopurinol also interferes with de novo pyrimidine nucleotide synthesis by inhibiting orotidine 5'-phosphate decarboxylase. Secondary orotic aciduria and orotidinuria result. Orotic acid is highly insoluble and could form a heavy sediment of urinary crystals; however, the increased excretion of orotic acid and orotidine rarely exceeds 10% of the total pyrimidines synthesized by the body. In addition, enhanced conversion of uridine to uridine 5'-monophosphate usually occurs and, therefore, this partial inhibition of pyrimidine synthesis is considered innocuous.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
Allopurinol may also inhibit hepatic microsomal enzymes. Allopurinol is not cytotoxic and has no effect on transplantable tumors. The drug has no analgesic, anti-inflammatory, or uricosuric activity.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
BUILDING BLOCK


