

X


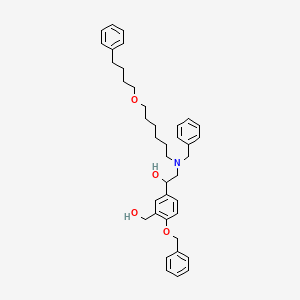

1. 943969-38-8
2. Dtxsid901115497
3. Alpha1-[[[6-(4-phenylbutoxy)hexyl](phenylmethyl)amino]methyl]-4-(phenylmethoxy)-1,3-benzenedimethanol
| Molecular Weight | 595.8 g/mol |
|---|---|
| Molecular Formula | C39H49NO4 |
| XLogP3 | 7.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 21 |
| Exact Mass | 595.36615904 g/mol |
| Monoisotopic Mass | 595.36615904 g/mol |
| Topological Polar Surface Area | 62.2 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 688 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs. 







