



1. 885477-83-8
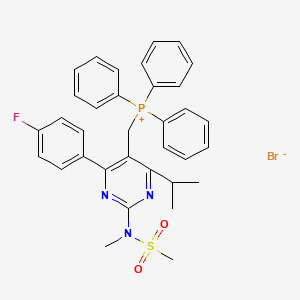
2. [[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]methyl]triphenylphosphonium Bromide
3. [4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]methyl-triphenylphosphanium;bromide
4. ((4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)-pyrimidin-5-yl)methyl)triphenylphosphonium Bromide
5. Phosphonium, [[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]methyl]triphenyl-, Bromide
6. Rosuvastatin Triphenylphosphonium Bromide
7. Schembl426278
8. Amy3281
9. Dtxsid70459687
10. Bcp10930
11. Mfcd12911893
12. Akos015896420
13. [4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]methyl-triphenylphosphanium,bromide
14. Ac-30583
15. Phosphonium, [[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]me
16. Cs-0154307
17. Ft-0656492
18. A24856
19. D82377
20. ((4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)methyl)triphenylphosphoniumbromide
21. {[4-(4-fluorophenyl)-2-[(methanesulfonyl)(methyl)amino]-6-(propan-2-yl)pyrimidin-5-yl]methyl}(triphenyl)phosphanium Bromide
22. Triphenyl[4-(4-fluorophenyl)-6-isopropyl-2-[(2-n-methyl-n-methylsulfonyl)amino]pyrimidine-5-yl-methyl]phosphine Bromine
| Molecular Weight | 678.6 g/mol |
|---|---|
| Molecular Formula | C34H34BrFN3O2PS |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 677.12768 g/mol |
| Monoisotopic Mass | 677.12768 g/mol |
| Topological Polar Surface Area | 71.5 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 889 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |









