


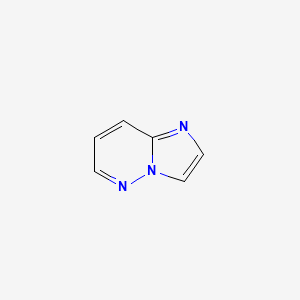

1. Imidazo(1,2-b)pyridazine
1. 766-55-2
2. Imidazo(1,2-b)pyridazine
3. 146233-39-8
4. Hc9c5rh4hc
5. Imidazo[1,2-b]pyridazine, Methanone Deriv
6. Mfcd07782103
7. Imdazo1,2-bpyridazine
8. Imidazo-[1,2-b]pyridazine
9. Unii-hc9c5rh4hc
10. Imidazol[1,2-b]pyridazine
11. 1,5-diazaindolizine
12. Schembl46494
13. 1,3a,4-triazaindene
14. Imidazo[1,2]pyridazine
15. Dtxsid10227408
16. Act02028
17. Bcp22884
18. Zinc5117297
19. Akos005254562
20. Ab42764
21. Ac-5585
22. Cs-w013583
23. Ds-0584
24. Ps-3092
25. Imidazo[1,2-b]pyridazine,methanone Deriv
26. Sy008983
27. Db-005439
28. Am20080351
29. Ft-0601104
30. Ft-0645177
31. I0742
32. 766i552
33. A838782
34. Q-100820
35. Methanone,[2-(1,1-dimethylethyl)imidazo[1,2-b]pyridazin-6-yl]phenyl-
| Molecular Weight | 119.12 g/mol |
|---|---|
| Molecular Formula | C6H5N3 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 119.048347172 g/mol |
| Monoisotopic Mass | 119.048347172 g/mol |
| Topological Polar Surface Area | 30.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 105 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




