



1. 51388-20-6
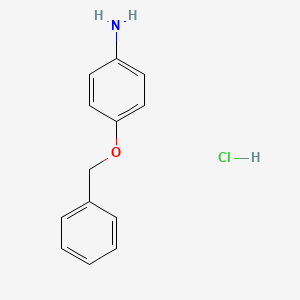
2. 4-(benzyloxy)aniline Hydrochloride
3. 4-benzyloxyaniline Hcl
4. 4-benzyloxyaniline, Hcl
5. 4-phenylmethoxyaniline;hydrochloride
6. Benzenamine, 4-(phenylmethoxy)-, Hydrochloride
7. Mfcd00012995
8. 4-(phenylmethoxy)aniline Hydrochloride
9. Einecs 257-170-6
10. [4-(benzyloxy)phenyl]amine Hydrochloride
11. Ai3-52569
12. Schembl371743
13. 4-benzyloxyanilinehydrochloride
14. P-benzyloxyaniline Hydrochloride
15. Dtxsid20199389
16. P-(benzyloxy)aniline Hydrochloride
17. Act00151
18. 4-benzyloxy-phenylamine Hydrochloride
19. 4-benzyloxyaniline Hydrochloride Salt
20. Ac7908
21. Akos005287354
22. Cs-w014671
23. Ps-7655
24. 4-aminophenyl Benzyl Ether Hydrochloride
25. 4-benzyloxyaniline Hydrochloric Acid Salt
26. 4-(benzyloxy)aniline Hydrochloride, 98%
27. 4-[(phenylmethyl)oxy]aniline Hydrochloride
28. Db-071347
29. Am20060650
30. Ft-0617659
31. Ft-0617660
32. B-1400
33. 388b206
34. A828565
35. Sr-01000597195
36. Sr-01000597195-1
37. F0850-6734
| Molecular Weight | 235.71 g/mol |
|---|---|
| Molecular Formula | C13H14ClNO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 235.0763918 g/mol |
| Monoisotopic Mass | 235.0763918 g/mol |
| Topological Polar Surface Area | 35.2 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 169 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |




