

X

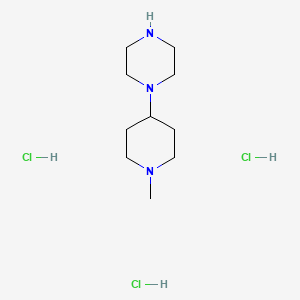

1. 349535-15-5
2. 1-(1-methylpiperidin-4-yl)piperazine 3hcl
3. Cs-0145450
4. 1-(1-methylpiperidin-4-yl)piperazine;trihydrochloride
| Molecular Weight | 292.7 g/mol |
|---|---|
| Molecular Formula | C10H24Cl3N3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 18.5 |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 146 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |



