



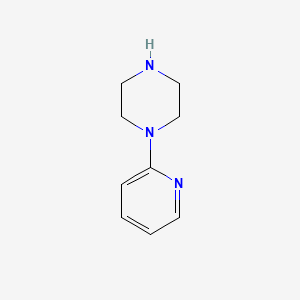

1. 1-(2-pyridinyl)piperazine
2. 1-(2-pyridinyl)piperazine, Sodium Salt
1. 34803-66-2
2. 1-(pyridin-2-yl)piperazine
3. 1-(2-pyridinyl)piperazine
4. 1-pyridin-2-yl-piperazine
5. 1-pyridin-2-ylpiperazine
6. Piperazine, 1-(2-pyridinyl)-
7. 2-pyridylpiperazine
8. N-(2-pyridyl)piperazine
9. Mfcd00006216
10. 2-(piperazin-1-yl)pyridine
11. N-pyridin-2-ylpiperazine
12. 4-(2-pyridyl)piperazine
13. 5io1hzp7zn
14. 4-(2-pyridinyl)piperazine
15. Chembl18094
16. Nsc-137781
17. Pyridinylpiperazine
18. Nsc-26624
19. 2-(1-piperazinyl)pyridine
20. Pyridylpiperazine
21. Pyridyl Piperazine
22. Einecs 252-220-3
23. 2-piperazinopyridine
24. 2-piperizinopyridine
25. 2-pyridyl-piperazine
26. Nsc137781
27. 2-piperazinylpyridine
28. Nsc 137781
29. (2-pyridyl) Piperazine
30. Unii-5io1hzp7zn
31. (pyridin-2-yl)piperazine
32. N-(2-pyridyl) Piperazine
33. 1-(2-pyridyl) Piperazine
34. 1-(2-pyridyl)-piperazine
35. 1-pyridin-2-yl Piperazine
36. 4-pyridin-2-yl-piperazine
37. 1-(2-pyridyl) Piperizine
38. Schembl7900
39. N-(pyridin-2-yl)piperazine
40. 1-(2-pyridinyl)-piperazine
41. 1-(pyridine-2-yl)piperazine
42. Bidd:gt0511
43. 1-(pyridin-2-yl)-piperazine
44. 4-(pyridin-2-yl)-piperazine
45. 1-(2-pyridinyl)piperazine #
46. Dtxsid90188341
47. 1-(2-pyridyl)piperazine, 98%
48. Act09575
49. Albb-032938
50. 1-(2-pyridyl)piperazine, >=99%
51. Bdbm50026634
52. Stk796767
53. Zinc19014810
54. Akos000266171
55. Piperazine, 1-(2-pyridyl)-
56. Ab00623
57. Am62509
58. Cs-w001990
59. Lf-0569
60. Wt81540
61. Sy012687
62. Db-027248
63. A6107
64. Bb 0218368
65. C3274
66. Ft-0605567
67. P1033
68. Q-102292
69. Q7263585
70. Brd-k72135511-001-01-2
71. F2169-0995
| Molecular Weight | 163.22 g/mol |
|---|---|
| Molecular Formula | C9H13N3 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 163.110947427 g/mol |
| Monoisotopic Mass | 163.110947427 g/mol |
| Topological Polar Surface Area | 28.2 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 132 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.  Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs. 













