
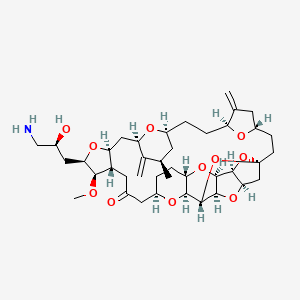

1. 2-(3-amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9h,15h-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopentacosin-5-(4h)-one
1. 253128-41-5
2. Eribuline
3. Eribulina
4. Chebi:63587
5. Er 086526
6. E-7389 Free Base
7. Lr24g6354g
8. 2-(3-amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9h,15h-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopentacosin-5-(4h)-one
9. Dtxsid101009321
10. Nsc-707389
11. B-1939
12. Eribulinum
13. L01xx41
14. Dtxcid701436148
15. Er-86526
16. Er086526
17. Eribulin [inn]
18. (1s,3s,6s,9s,12s,14r,16r,18s,20r,21r,22s,26r,29s,31r,32s,33r,35r,36s)-20-[(2s)-3-amino-2-hydroxypropyl]-21-methoxy-14-methyl-8,15-dimethylidene-2,19,30,34,37,39,40,41-octaoxanonacyclo[24.9.2.13,32.13,33.16,9.112,16.018,22.029,36.031,35]hentetracontan-24-one
19. Er-086526
20. (2r,3r,3as,7r,8as,9s,10ar,11s,12r,13ar,13bs,15s,18s,21s,24s,26r,28r,29as)-2-[(2s)-3-amino-2-hydroxypropyl]hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)-11,15:18,21:24,28-triepoxy-7,9-ethano-12,15-methano-9h,15h-furo[3,2-i]furo[2',3':5,6]pyrano[4,3-b][1,4]dioxacyclopentacosin-5(4h)-one
21. (1s,3s,6s,9s,12s,14r,16r,18s,20r,21r,22s,26r,29s,31r,32s,35r,36s)-20-[(2s)-3-amino-2-hydroxypropyl]-21-methoxy-14-methyl-8,15-bis(methylene)-2,19,30,34,37,39,40,41-octaoxanonacyclo[24.9.2.1(3,32).1(3,33).1(6,9).1(12,16).0(18,22).0(29,36).0(31,35)]hentetracontan-24-one
22. Eribulin Free
23. Unii-lr24g6354g
24. (1s,3s,6s,9s,12s,14r,16r,18s,20r,21r,22s,26r,29s,31r,32s,33r,35r,36s)-20-[(2s)-3-amino-2-hydroxypropyl]-21-methoxy-14-methyl-8,15-dimethylidene-2,19,30,34,37,39,40,41-octaoxanonacyclo[24.9.2.1~3,32~.1~3,33~.1~6,9~.1~12,16~.0~18,22~.0~29,36~.0~31,35~]hentetracontan-24-one (non-preferred Name)
25. Eribulin [mi]
26. Eribulin [vandf]
27. Eribulin [mart.]
28. Eribulin [who-dd]
29. B 1939
30. E7389-lf
31. Gtpl6813
32. Orb1707961
33. Chembl1683590
34. Schembl15783821
35. Ex-a4873d
36. Ufnvpogxiszxjd-jbqzkeiosa-n
37. Glxc-10730
38. Akos040740784
39. At36513
40. Db08871
41. Ncgc00510497-02
42. Bp-29357
43. Da-52974
44. Hy-13442
45. Ms-31267
46. Q408717
47. 2-[4-[[(2r)-2-aminobutyl]amino]-2-quinazolinyl]-4-chloro-phenol
48. 11,15:18,21:24,28-triepoxy-7,9-ethano-12,15-methano-9h,15h-furo[3,2-i]furo[2',3':5,6]pyrano[4,3-b][1,4]dioxacyclopentacosin-5(4h)-one, 2-[(2s)-3-amino-2-hydroxypropyl]hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)-, (2r,3r,3as,7r,8as,9s,10ar,11s,12r,13ar,13bs,15s,18s,21s,24s,26r,28r,29as)-
| Molecular Weight | 729.9 g/mol |
|---|---|
| Molecular Formula | C40H59NO11 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 4 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 146 |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 1380 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic cancer.
FDA Label
Halaven monotherapy is indicated for the treatment of patients with locally advanced or metastatic breast cancer who have progressed after at least one chemotherapeutic regimens for advanced disease (see section 5. 1). Prior therapy should have included an anthracycline and a taxane unless patients were not suitable for these treatments. Halaven is indicated for the treatment of adult patients with unresectable liposarcoma who have received prior anthracycline containing therapy (unless unsuitable) for advanced or metastatic disease (see section 5. 1).
Treatment of soft tissue sarcoma
L01XX41
L01XX41
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (2021) DOI:10.1021/acsenvironau.1c00008. List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX41 - Eribulin
Route of Elimination
Eribulin is eliminated primarily in feces unchanged.
Volume of Distribution
43 L/m2 to 114 L/m2
Clearance
1.16 L/hr/m2 to 2.42 L/hr/m2 (dose range of 0.25 mg/m2 to 4.0 mg/m2). [FDA]
There are no major human metabolites of eribulin, CYP3A4 negligibly metabolizes eribulin in vitro.
about 40 hours
Eribulin inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates. Eribulin exerts its effects via a tubulin-based antimitotic mechanism leading to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage. [FDA]
BUILDING BLOCK


