

X


1. 2161380-87-4
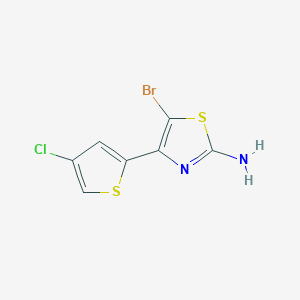
2. 5-bromo-4-(4-chlorothiophen-2-yl)thiazol-2-amine (avatrombopag Impurity)
3. Schembl19966961
4. Amy40538
5. Cs-0377234
6. F83912
| Molecular Weight | 295.6 g/mol |
|---|---|
| Molecular Formula | C7H4BrClN2S2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 95.4 |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 197 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





