



1. 1985607-83-7
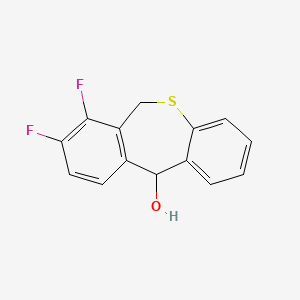
2. 7,8-difluoro-6,11-dihydrobenzo[c][1]benzothiepin-11-ol
3. 7,8-difluoro-6,11-dihydro-dibenzo[b,e]thiepin-11-ol
4. Baloxavir Impurity 8
5. Schembl20924690
6. 7,8-difluoro-11-hydroxy-6,11-dihydrodibenzo[b,e]thiepine
7. Dtxsid901227086
8. Amy16546
9. Bcp30161
10. Ex-a2712
11. Mfcd31693069
12. Akos037651023
13. Ac-31354
14. Cs-15728
15. Sy226916
16. Cs-0040733
17. J3.610.381g
18. D72680
19. Zofluza Intermediate;7,8-difluoro-6,11-dihydro-dibenzo[b,e]thiepin-11-ol
| Molecular Weight | 264.29 g/mol |
|---|---|
| Molecular Formula | C14H10F2OS |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 264.04204244 g/mol |
| Monoisotopic Mass | 264.04204244 g/mol |
| Topological Polar Surface Area | 45.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



