

X


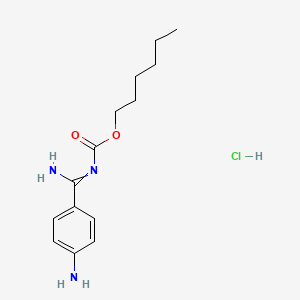

1. 1307233-93-7
2. Hexyl N-[amino-(4-aminophenyl)methylidene]carbamate;hydrochloride
3. Hexyl (amino(4-aminophenyl)methylene)carbamate Hydrochloride
4. Hexyl ((4-aminophenyl)(imino)methyl)carbamate Hydrochloride
| Molecular Weight | 299.79 g/mol |
|---|---|
| Molecular Formula | C14H22ClN3O2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 90.7 |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 297 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 1 |
| Covalently Bonded Unit Count | 2 |



