
1. 4-nitroaniline Monohydrochloride
2. 4-nitroaniline Sulfate (2:1)
3. 4-nitroaniline, Mercury (2+) Salt (2:1)
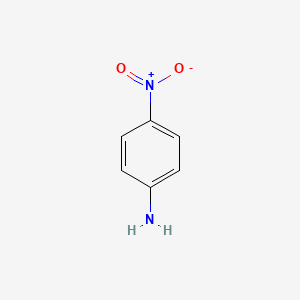
4. P-nitroaniline
5. Para-nitroaniline
6. Paranitronaniline
1. P-nitroaniline
2. 100-01-6
3. 4-nitrobenzenamine
4. P-aminonitrobenzene
5. P-nitraniline
6. P-nitrophenylamine
7. 4-nitraniline
8. 1-amino-4-nitrobenzene
9. Benzenamine, 4-nitro-
10. Aniline, P-nitro-
11. Developer P
12. Aniline, 4-nitro-
13. Para-nitroaniline
14. Azoamine Red Zh
15. Nitrazol Cf Extra
16. Devol Red Gg
17. Fast Red P Base
18. Fast Red P Salt
19. Fast Red Base Gg
20. Fast Red Gg Base
21. Fast Red Gg Salt
22. Fast Red Mp Base
23. Fast Red Salt Gg
24. Diazo Fast Red Gg
25. Red 2g Base
26. Fast Red 2g Base
27. Fast Red 2g Salt
28. Fast Red Base 2j
29. Fast Red Salt 2j
30. Azofix Red Gg Salt
31. C.i. Developer 17
32. Naphtoelan Red Gg Base
33. Azoic Diazo Component 37
34. Paranitroaniline
35. P-nitroanilina
36. Shinnippon Fast Red Gg Base
37. Rcra Waste Number P077
38. C.i. Azoic Diazo Component 37
39. P-nitro Aniline
40. Nci-c60786
41. Nsc 9797
42. C.i. 37035
43. 4-nitro-aniline
44. 1mrq0qzg7g
45. Dtxsid8020961
46. Chebi:17064
47. Nsc-9797
48. Ncgc00091426-02
49. 4-nitrobenzeneamine
50. 4-aminonitrobenzene
51. Ci Developer 17
52. Para-aminonitrobenzene
53. P-nitroanilina [polish]
54. Pna (van)
55. 4-nitro-phenylamine
56. 4-nitrophenylamine
57. Nitroaniline, P-
58. Azoamine Red 2h
59. Ci Azoic Diazo Component 37
60. Ccris 1184
61. Hsdb 1156
62. Benzen-2,3,5,6-d4-amine-d2, 4-nitro-
63. Einecs 202-810-1
64. Para Nitro Aniline
65. Unii-1mrq0qzg7g
66. Mfcd00007858
67. Rcra Waste No. P077
68. Ci 37035
69. P-nitro-aniline
70. Para-nitroanilin
71. Ai3-08926
72. 4-nitro Aniline
73. P-nitroaniline, Solid
74. (4-nitrophenyl)-amine
75. 4-nitroaniline, 99%
76. 4-nitrobenzeneamine Anion
77. Dsstox_cid_961
78. Wln: Zr Dnw
79. Ec 202-810-1
80. 4-nitroaniline, Crystalline
81. 4-nitroaniline, >=99%
82. Dsstox_rid_75889
83. Nciopen2_002864
84. P-nitroaniline [mi]
85. Dsstox_gsid_20961
86. Schembl16451
87. Mls002454441
88. Chembl14282
89. 4-nitroaniline [hsdb]
90. 4-nitro-3-chloro Benzoic Acid
91. Schembl10163408
92. Nsc9797
93. Hms3039b17
94. 4-nitroaniline, Analytical Standard
95. Str00350
96. Zinc3860644
97. Tox21_400031
98. Stk301653
99. Akos000119131
100. Akos025293369
101. P-nitroaniline [un1661] [poison]
102. Ncgc00091426-01
103. Ncgc00091426-03
104. Cas-100-01-6
105. Smr001372024
106. Ft-0650287
107. N0119
108. 00n016
109. C02126
110. Ae-641/01643038
111. Q419842
112. Q-200503
113. Z275127984
| Molecular Weight | 138.12 g/mol |
|---|---|
| Molecular Formula | C6H6N2O2 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 138.042927438 g/mol |
| Monoisotopic Mass | 138.042927438 g/mol |
| Topological Polar Surface Area | 71.8 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 124 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): IN VET MEDICINE FOR POULTRY
SRI
It is absorbed through the skin.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2468
... Absorbed ... by inhalation of dust or vapor.
International Labour Office. Encyclopedia of Occupational Health and Safety. Vols. I&II. Geneva, Switzerland: International Labour Office, 1983., p. 145
The disposition of 14(C)-labeled p-nitroaniline was studied in male rats following oral or iv admin. The clearance of 14(C) p-nitroaniline-derived radioactivity from various tissues was rapid and followed a 2-component decay curve. The whole-body half-life of p-nitroaniline was approximately 1 hr. Within 3 days, clearance of p-nitroaniline-derived radioactivity from the body was almost complete. 14(C) p-nitroaniline was rapidly cleared by metabolism to 9 metabolites which were excreted primarily in the urine and to a lesser extent in the feces. Most (56%) of the urinary radioactivity was in the form of sulfate conjugates of 2 metabolites of p-nitroaniline.
PMID:6745538 Chopade HM, Matthews HB; Fundam Appl Toxicol 4 (3): 485-93 (1984)
The percutaneous absorption of nitrobenzene, p-nitroaniline, 2,4-dinitrochlorobenzene, 2-nitro-p-phenylenediamine, and 4-amino-2-nitrophenol was studied in vivo and in vitro. The compounds were applied to shaved abdominal skins of Rhesus-monkeys at a concentration of 4 ug/sq cm. Five day urine samples were collected and analyzed for the compounds. Radiolabeled nitrobenzene, p-nitroaniline, 2-4-dinitrochlorobenzene, 2-nitro-p-phenylenediamine, or 4-amino-2-nitrophenol were applied to excised human skin at a dose of 4 ug/sq cm using a diffusion cell technique. Penetration of radioactivity through the skin was monitored for the next 24 hours. The compounds rapidly penetrated excised human skin, the greatest penetration occurring in the first 2 hours after exposure. The ability of the compounds to penetrate human skin ranked in decreasing order of permeability and corrected for evaporation was 4-amino-2-nitrophenol, nitrobenzene, p-nitroaniline, 2,4-dinitrochlorobenzene, and 2-nitro-p-phenylenediamine. The corresponding rank for penetration of monkey skin was 4-amino-2-nitrophenol, p-nitroaniline, 2,4-dinitrochlorobenzene, 2-nitro-p-phenylenediamine, and nitrobenzene.
Bronaugh RL, Maibach HI; Toxicity of Nitroaromatic Compounds 141-8 (1985)
Given orally or ip in doses of 20-40 mg/kg in rats, analysis of 24-hr urine samples showed that 4-nitroaniline was excreted partly unchanged & also as 4-phenylenediamine & 2-amino-5-nitrophenol...
PMID:4868295 MATE C ET AL; FOOD COSMET TOXICOL 5 (5): 657-63 (1967)
The principal rat liver microsomal metabolite of 4-nitroaniline was isolated by high performance liquid chromatography and characterized as 2-amino-5-nitrophenol. Pretreatment of rats with phenobarbital and 3-methylcholanthrene increased the rate of conversion of 4-nitroaniline to 2-amino-5-nitrophenol by 2- and 4-fold, respectively.
PMID:6144483 Anderson MM et al; Drug Metab Dispos 12 (2): 179-85 (1984)
The metabolism of radiolabeled dinitrobenzene isomers was compared in hepatocytes and hepatic subcellular fractions isolated from male rats. Under aerobic conditions, reduction was the major metabolic pathway for m-dinitrobenzene and p-dinitrobenzene in hepatocytes with m-nitroaniline and p-nitroaniline accounting for 74.0 and 81.0% respectively, of the radioactivity present after a 30-minute incubation. The major metabolite of o-nitrobenzene in similar incubations was S-(2-nitrophenyl)glutathione which represented 48.1% of the total radioactivity. o-Nitroaniline accounted for 29.5% of the radioactivity.
PMID:2867868 Cossum PA, Rickert DE; Drug Metab Dispos 13 (6): 664-8 (1985)
14(C) p-nitroaniline was rapidly cleared by metabolism to 9 metabolites which were excreted primarily in the urine and to a lesser extent in the feces. Most (56%) of the urinary radioactivity was in the form of sulfate conjugates of 2 metabolites of p-nitroaniline.
PMID:6745538 Chopade HM, Matthews HB; Fundam Appl Toxicol 4 (3): 485-93 (1984)
The whole-body half-life of p-nitroaniline was approximately 1 hr.
PMID:6745538 Chopade HM, Matthews HB; Fundam Appl Toxicol 4 (3): 485-93 (1984)
... To assess further the role of the 4-substituent in methaemoglobinaemia, the toxicity of a series of 4-substituted aniline derivatives was also studied. Of the anilines studied, 4-nitroaniline caused the most methemoglobin (36.5 +/- 8.0%), while aniline caused the least (0.3 +/- 0.5%). Overall, there was a significant correlation (r2 = 0.83) between the hemotoxicity and the Hammett constant, sigma(p), suggesting that it is the electron-withdrawing properties of the substituent that influence the methemoglobin formation. ...
PMID:9020194 Mahmud R et al; Toxicology 117 (1): 1-11 (1997)
The relative mutagenic activities of aminoanilines have been attempted to be related to parameters reflecting potential for n-hydroxylation and stability of the arylnitrenium ions. Both chloro and the nitro groups deactivate the amine group to n-hydroxylation and the ring to epoxidation, & no active products from cytochrome p450 would be predicted. The activity of the nitro derivatives is presumed to be due to transformation of the nitro group itself to an active mutagenic species by other enzyme systems. /Aminoanilines/
PMID:448254 LOEW GH ET AL; J ENVIRON PATHOL TOXICOL 2 (4): 1069-78 (1979)
BUILDING BLOCK


