




1. 41340-36-7
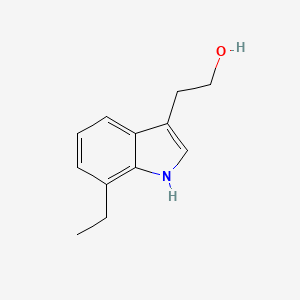
2. 7-ethyltryptophol
3. 7-ethyl Tryptophol
4. 7-ethyl-3-indoleethanol
5. 7-ethyl-1h-indole-3-ethanol
6. 1h-indole-3-ethanol, 7-ethyl-
7. 2-(7-ethyl-1h-indol-3-yl)-ethanol
8. 7-ethyl-3-(2-hydroxyethyl)indole
9. Jj4lb8km8o
10. 2-(7-ethylindol-3-yl)ethanol
11. Ccris 5406
12. Rak-801
13. Brn 1074382
14. Etodolac Impurity H
15. 2-(7-ethyl-1h-indol-3-yl)ethan-1-ol
16. 7-ethylindole-3-ethanol
17. Unii-jj4lb8km8o
18. 3-(7-ethylindole)ethanol
19. Ec 431-020-1
20. Dsstox_cid_31530
21. Dsstox_rid_97415
22. Dsstox_gsid_57741
23. Schembl4803022
24. Chembl3182675
25. Dtxsid1057741
26. Zinc2383204
27. Tox21_113757
28. 7-ethyl-3-(2-hydroxy Ethyl)indole
29. Mfcd01718805
30. Akos005259419
31. 2-(3a-ethyl-3ah-indol-3-yl)ethanol
32. Ac-7753
33. Sb37105
34. Ncgc00253629-01
35. As-12049
36. Cas-41340-36-7
37. Db-049741
38. E0824
39. Ft-0639708
40. E-9050
41. F20442
42. 340e367
43. A825522
| Molecular Weight | 189.25 g/mol |
|---|---|
| Molecular Formula | C12H15NO |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 189.115364102 g/mol |
| Monoisotopic Mass | 189.115364102 g/mol |
| Topological Polar Surface Area | 36 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 183 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




