


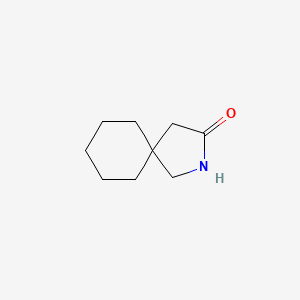

1. 1-(aminomethyl)cyclohexaneacetic Acid Lactam
2. 8-aza-spiro(5,4)decan-9-on
3. Gabapentin-lactam
1. 64744-50-9
2. Gabapentin-lactam
3. 4,4-pentamethylene-2-pyrrolidinone
4. 3,3-pentamethylene-4-butyrolactam
5. Gabapentin Lactam
6. 2-azaspiro-[4,5]-decan-3-one
7. Gabapentin Related Compound A
8. 2-azaspiro(4,5)decan-3-one
9. 2-azaspiro(4.5)decan-3-one
10. 2-aza-spiro[4.5]decan-3-one
11. 4,4-pentamethylene-2-pyrrolidone
12. Mfcd00177938
13. 1-(aminomethyl)cyclohexaneacetic Acid Lactam
14. 0ip6953295
15. 3-azaspiro[4.5]decan-2-one
16. 8-aza-spiro(5,4)decan-9-on
17. 8-aza-spiro[5,4]decan-9-on
18. Brn 0119500
19. Unii-0ip6953295
20. Beta,beta-pentamethylen-gamma-butyrolactam [german]
21. Beta,beta-pentamethylen-gamma-butyrolactam
22. Ec 451-630-1
23. 5-21-07-00071 (beilstein Handbook Reference)
24. Schembl483007
25. A Gabapentin Dehydration Product
26. Chembl3115477
27. 2-aza-spiro[4,5]decan-3-one
28. Dtxsid40215070
29. Chebi:182289
30. 3,3-pentamethylene-4-butyrolactum
31. Act02804
32. Albb-013331
33. Zinc1845277
34. 2-pyrrolidone, 4,4-pentamethylene-
35. Ac1536
36. Stl450936
37. Akos005169332
38. Gabapentin Lactam [nflis-drug]
39. Ac-8435
40. Ks-5267
41. 2-pyrrolidone,4,4-pentamethylene
42. Sy006466
43. 4,4-pentamethylene-2-pyrrolidinone, 96%
44. Beta, Beta-pentamethylen-gamma-butyrolactam
45. Gabapentin Impurity A [ep Impurity]
46. Cs-0096868
47. Ft-0640970
48. P1695
49. En300-94425
50. 44g509
51. Gabapentin Related Compound A [usp-rs]
52. Gabapentin-lactam 100 Microg/ml In Acetonitrile
53. .beta.,.beta.-pentamethylen-.gamma.-butyrolactam
54. Gabapentin Related Compound A [usp Impurity]
55. Q-200416
56. Q27236831
57. Z1270207933
58. 2-bromo-2-(4-methoxy-phenyl-hydrazono)-aceticacidethylester
59. Gabapentin Ep Impurity A; Gabapentin Usp Related Compound A
60. 2-azaspiro[4.5]decan-3-one (3,3-pentamethylene-4-butyrolactam)
61. Gabapentin Impurity A, European Pharmacopoeia (ep) Reference Standard
62. Gabapentin Related Compound A, United States Pharmacopeia (usp) Reference Standard
63. 4,4-pentamethylene-2-pyrrolidone(gabapentin Related Compound A)(gabapentin Impurity A), Pharmaceutical Secondary Standard; Certified Reference Material
64. Gabapentin Usp Related Compound A; Gabapentin Lactam; 3-azaspiro-[4,5]decan-3-one; 3,3-pentamethylene-5-butyrolactam; 4,4-pentamethylene-2-pyrrolidinone
| Molecular Weight | 153.22 g/mol |
|---|---|
| Molecular Formula | C9H15NO |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 153.115364102 g/mol |
| Monoisotopic Mass | 153.115364102 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




