



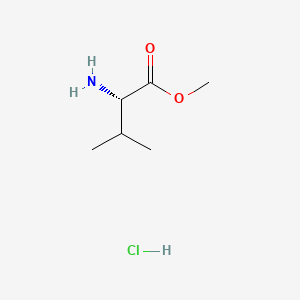

1. 6306-52-1
2. H-val-ome.hcl
3. Methyl Valinate Hydrochloride
4. (s)-methyl 2-amino-3-methylbutanoate Hydrochloride
5. L-valine Methyl Ester Hcl
6. Methyl L-valinate Hydrochloride
7. H-l-val-ome Hcl
8. L-valine Methyl Ester, Hcl
9. L-valine, Methyl Ester, Hydrochloride
10. Valine Methyl Ester Hydrochloride
11. H-val-ome Hydrochloride
12. (s)-2-amino-3-methyl-butyric Acid Methyl Ester Hydrochloride
13. Methyl (2s)-2-amino-3-methylbutanoate;hydrochloride
14. H-val-ome Hcl
15. L-valine Methylester Hcl
16. L-valine Methyl-ester Hcl
17. L-methyl Valinate Hydrochloride
18. Methyl (2s)-2-amino-3-methylbutanoate Hydrochloride
19. Mfcd00012497
20. Methyl Valinate Hcl
21. (s)-2-amino-3-methyl-butyric Acid Methyl Esterhydrochloride
22. Einecs 228-620-9
23. H-val-ome?cl
24. Val-ome Hydrochloride
25. H-val-ome. Hcl
26. H-val-ome⋅hcl
27. H-val-och3.hcl
28. Schembl261172
29. Valine Methylester Hydrochloride
30. Valin Methyl Ester Hydrochloride
31. Chembl1221961
32. Dtxsid60212389
33. L-valine Methylester Hydrochloride
34. L-valine Methyl Ester Hydrochoride
35. L Valine Methyl Ester Hydrochloride
36. Act08510
37. Bcp11286
38. Str02995
39. Nsc 22920
40. (l)-valine Methyl Ester Hydrochloride
41. (s)-valine Methyl Ester Hydrochloride
42. Akos015845881
43. Akos015892711
44. Ac-6863
45. Cs-w010793
46. Hy-w010077
47. Nsc 197198
48. L-(+)-valine Methyl Ester Hydrochloride
49. L-valine Methyl Ester Hydrochloride, 99%
50. Db-029990
51. Am20100557
52. V0056
53. M02951
54. 306v521
55. J-300307
56. L-2-aminoisovaleric Acid Methyl Ester Hydrochloride
57. Q-101859
| Molecular Weight | 167.63 g/mol |
|---|---|
| Molecular Formula | C6H14ClNO2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 167.0713064 g/mol |
| Monoisotopic Mass | 167.0713064 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 101 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |











