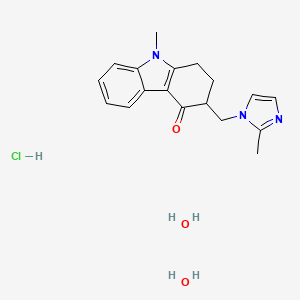

1. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-
2. Dihydrate, Ondansetron Monohydrochloride
3. Gr 38032f
4. Gr-38032f
5. Gr38032f
6. Hydrochloride, Ondansetron
7. Monohydrochloride Dihydrate, Ondansetron
8. Monohydrochloride, Ondansetron
9. Odt, Zofran
10. Ondansetron
11. Ondansetron Hydrochloride
12. Ondansetron Monohydrochloride
13. Ondansetron Monohydrochloride Dihydrate
14. Ondansetron, (+,-)-isomer
15. Ondansetron, (r)-isomer
16. Ondansetron, (s)-isomer
17. Sn 307
18. Sn-307
19. Sn307
20. Zofran
21. Zofran Odt
1. 103639-04-9
2. Zofran
3. Zophren
4. Ondansetron Hydrochloride Hydrate
5. Gr 38032f
6. 99614-01-4
7. Sn 307
8. Ondansetron Hcl Dihydrate
9. Ondansetron Monohydrochloride
10. Gr-38032f
11. Sn-307
12. Odansetron Hydrochloride
13. Zofran Odt
14. Ondansetron (hydrochloride Dihydrate)
15. Nmh84ozk2b
16. Ondansetron (as Hydrochloride)
17. 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1h-carbazol-4-one;dihydrate;hydrochloride
18. Gr 38032
19. Gr-c507/75
20. Unii-nmh84ozk2b
21. Setofilm
22. Setrodon
23. Zensana
24. Zofrene
25. Yatrox
26. Zofran Zydis
27. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-2,3-dihydro-1h-carbazol-4(9h)-one Hydrochloride Dihydrate
28. Mfcd00374371
29. Zofran (tn)
30. Ondansetron Hydrochloride [usan:usp:jan]
31. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-1,2,3,9-tetrahydro-4h-carbazol-4-one Hydrochloride Dihydrate
32. Ncgc00016968-01
33. Dsstox_cid_28783
34. Dsstox_rid_83052
35. Cas-103639-04-9
36. Dsstox_gsid_48857
37. (+-)-2,3-dihydro-9-methyl-3-((2-methylimidazol-1-yl)methyl)carbazol-4(1h)-one Monohydrochloride Dihydrate
38. Ondansetron Hydrochloride,(s)
39. Chebi:7774
40. Ondansetronhydrochloridedihydrate
41. Chembl3186492
42. Dtxsid9048857
43. Hy-b0002a
44. Rhb-102
45. Ondansetron (hydrochloride Hydrate)
46. Bcp21778
47. Tox21_113345
48. Ac-667
49. Gg-032
50. S4748
51. Ondansetron For Lc System Suitability
52. Akos015994673
53. Ondansetron For Tlc System Suitability
54. Bcp9001026
55. Ccg-268235
56. Cs-1716
57. Gr-c505/75
58. Ks-1091
59. To-2061
60. W39o049
61. Ondansetron Hydrochloride [usan]
62. Ondansetron Hydrochloride [mart.]
63. Ondansetron Hydrochloride [vandf]
64. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-, Monohydrochloride, (+-)-, Dihydrate
65. Ac-30904
66. Bo164178
67. Ondansetron Hydrochloride [usp-rs]
68. Ondansetron Hydrochloride Dihydrate- Bio-x
69. Db-040472
70. Ft-0631028
71. Ondansetron Hydrochloride Hydrate (jan/usp)
72. C71153
73. D00678
74. Ondansetron Hydrochloride [orange Book]
75. Ondansetron Hydrochloride Dihydrate [mi]
76. Ondansetron Hydrochloride Hydrate [jan]
77. Ondansetron Hydrochloride [usp Monograph]
78. Gr 38032;sn 307;nsc 665799
79. Ondansetron Hydrochloride Dihydrate [who-dd]
80. Q27284954
81. Ondansetron Hydrochloride Dihydrate [ep Monograph]
82. Ondansetron Hydrochloride Dihydrate, >=98% (hplc), Powder
83. Ondansetron For Lc System Suitability, European Pharmacopoeia (ep) Reference Standard
84. Ondansetron For Tlc System Suitability, European Pharmacopoeia (ep) Reference Standard
85. Ondansetron Hydrochloride Dihydrate, European Pharmacopoeia (ep) Reference Standard
86. Ondansetron Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
87. Ondansetron Hydrochloride, United States Pharmacopeia (usp) Reference Standard
88. (+/-)-2,3-dihydro-9-methyl-3-((2-methylimidazol-1-yl)methyl)carbazol-4(1h)-one Monohydrochloride Dihydrate
89. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-4h-carbazol-4-one Hydrochloride Dihydrate
90. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazole-1-yl)methyl]-4h-carbazol-4-one Hydrochloride Dihydrate
91. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-, Hydrochloride, Hydrate (1:1:2)
92. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-, Monohydrochloride, (+/-)-, Dihydrate
93. 4h-carbazol-4-one,1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-,monohydrochloride, Dihydrate
| Molecular Weight | 365.9 g/mol |
|---|---|
| Molecular Formula | C18H24ClN3O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 365.1506193 g/mol |
| Monoisotopic Mass | 365.1506193 g/mol |
| Topological Polar Surface Area | 41.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Serotonin 5-HT3 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT3 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT3 RECEPTOR AGONISTS. (See all compounds classified as Serotonin 5-HT3 Receptor Antagonists.)
MARKET PLACE

Reply
19 Aug 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
