




1. 482577-59-3
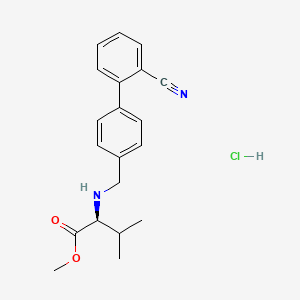
2. N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-l-valine Methyl Ester Hydrochloride
3. (s)-methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-methylbutanoate Hcl
4. N-(2'-cyanobiphenyl-4-ylmethyl)-l-valine Methyl Ester Hydrochloride
5. Methyl (2s)-2-[[4-(2-cyanophenyl)phenyl]methylamino]-3-methylbutanoate;hydrochloride
6. Schembl3526296
7. Dtxsid90587774
8. Bcp12021
9. Mfcd08460094
10. Akos015896156
11. Methyl ((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-l-valinate Hydrochloride
12. Cs-w014480
13. Hy-w013764
14. (s)-methyl 2-(((2`-cyano-[1,1`-biphenyl]-4-yl)methyl)amino)-3-methylbutanoate Hydrochloride
15. As-74409
16. F11554
17. N-(2'-cyanobiphenyl-4-ylmethyl)-l-valine Methyl Ester Hcl
18. N-(2\'-cyanobiphenyl-4-ylmethyl)-l-valine Methyl Ester Hydrochloride
19. N-[(2'-cyanobiphenyl-4-yl) Methyl]-(l)-valine Methyl Ester Hydrochloride
20. N-[(2'-cyanobiphenyl-4-yl)methyl]-(l)-valine Methyl Ester Hydrochloride
21. N-[(2'-cyanobiphenyl-4-yl)methyl]-(l)-valinemethyl Ester Hydrochloride
22. (s)-methyl 2-(((2-cyano-[1,1-biphenyl]-4-yl)methyl)amino)-3-methylbutanoate Hcl
23. L-valine,n-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-,methyl Ester,hydrochloride(1:1)
24. Methyl N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-l-valinate--hydrogen Chloride (1/1)
25. (s)-methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)-methyl)amino)-3-methylbutanoate Hydrochloride
26. Methyl (2s)-2-[({2'-cyano-[1,1'-biphenyl]-4-yl}methyl)amino]-3-methylbutanoate Hydrochloride
| Molecular Weight | 358.9 g/mol |
|---|---|
| Molecular Formula | C20H23ClN2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 358.1448057 g/mol |
| Monoisotopic Mass | 358.1448057 g/mol |
| Topological Polar Surface Area | 62.1 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 446 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |





