




1. 7682-20-4
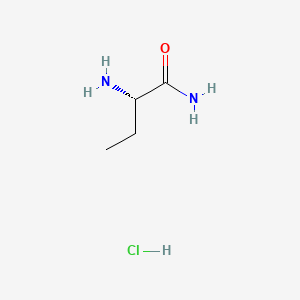
2. (s)-2-aminobutyramide Hydrochloride
3. H-abu-nh2 Hcl
4. (s)-2-aminobutanamide Hydrochloride
5. L-2-aminobutanamide Hydrochloride
6. (s)-(+)-2-aminobutanamide Hydrochloride
7. (2s)-2-aminobutanamide;hydrochloride
8. L-2-aminobutanamide, Hcl
9. Butanamide, 2-amino-, Monohydrochloride, (2s)-
10. L-homoalanylamide Hcl
11. 141978-61-2
12. 2c1v4527rj
13. Levetiracetam Related Compound B [usp-rs]
14. Butanamide, 2-amino-, Monohydrochloride, (s)-
15. Butanamide, 2-amino-, Hydrochloride (1:1), (2s)-
16. H-abu(2)-nh2 Hcl
17. (s)-2-aminobutanamide Hcl
18. (s)-2-amino Butanamide Hcl
19. (s)-2-amino-butanamide Hcl
20. Mfcd00136565
21. H-abu-nh2hcl
22. (s)-2-amino-butanamide Hydrochloride
23. S (+)-2-aminobutyramide Hydrochloride
24. L-2-aminobutanamide Hcl
25. Schembl373444
26. Unii-2c1v4527rj
27. L-homoalanylamide Hydrochloride
28. (s)-2-amino-butylactamide Hcl
29. Dtxsid10474839
30. S-2-aminobutanamide Hydrochloride
31. Act02761
32. Cs-b0864
33. H-abu-nh2 Hydrochloride, Aldrichcpr
34. Akos015910759
35. Ds-1209
36. Butanamide,2-amino-,hydrochloride(1:1)
37. Ac-23986
38. Am20100759
39. (s)-2-aminobutanamide Hydrochloride [usp-rs]
40. (s)-(+)-2-aminobutanamide Hydrochloride, 97%
41. 682a204
42. A935960
43. (2s)-2-aminobutanamide--hydrogen Chloride (1/1)
44. Butyramide, 2-amino-, Monohydrochloride, L-
45. Q-102786
46. Levetiracetam Related Compound B, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 138.59 g/mol |
|---|---|
| Molecular Formula | C4H11ClN2O |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 138.0559907 g/mol |
| Monoisotopic Mass | 138.0559907 g/mol |
| Topological Polar Surface Area | 69.1 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 72.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |



