




1. 201594-84-5
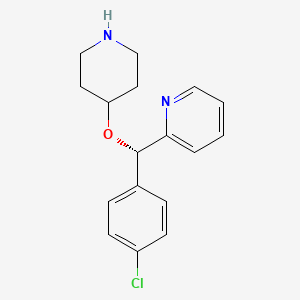
2. (s)-2-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine
3. Pyridine, 2-[(s)-(4-chlorophenyl)(4-piperidinyloxy)methyl]-
4. 2-[(s)-(4-chlorophenyl)-piperidin-4-yloxymethyl]pyridine
5. Pyridine,2-[(s)-(4-chlorophenyl)(4-piperidinyloxy)methyl]-
6. 2-[(s)-(4-chlorophenyl)-(4-piperidinyloxy)-methyl]-pyridine
7. Schembl2805296
8. Dtxsid70447428
9. (s)-(-)-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine
10. Amy21945
11. Bcp13376
12. Cs-b0348
13. Mfcd09955359
14. Zinc22007005
15. Akos015901675
16. Ac-6931
17. Ds-18255
18. 594c845
19. A879802
20. (s)-4-[(4-chlorophenyl) (2-pyridyl)methoxy]piperidine
21. (s)-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine
22. 4-[[(s)-4-chloro-alpha-(2-pyridyl)benzyl]oxy]piperidine
23. (s)-(-)-4-[(4-chlorophenyl) (2-pyridyl)methoxy]piperidine
24. 2-{(s)-(4-chlorophenyl)[(piperidin-4-yl)oxy]methyl}pyridine
| Molecular Weight | 302.8 g/mol |
|---|---|
| Molecular Formula | C17H19ClN2O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 302.1185909 g/mol |
| Monoisotopic Mass | 302.1185909 g/mol |
| Topological Polar Surface Area | 34.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 301 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





