


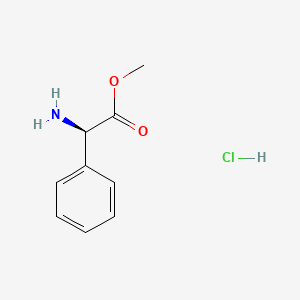

1. 19883-41-1
2. H-d-phg-ome Hcl
3. (r)-2-phenylglycine Methyl Ester Hydrochloride
4. (r)-(-)-2-phenylglycine Methyl Ester Hydrochloride
5. Methyl (2r)-2-amino-2-phenylacetate Hydrochloride
6. Methyl (2r)-2-amino-2-phenylacetate;hydrochloride
7. Mfcd00137487
8. D-alpha-phenylglycine Methyl Ester Hydrochloride
9. D-(-)-2-phenylglycine Methyl Ester Hydrochloride
10. Methyl (r)-2-amino-2-phenylacetate Hydrochloride
11. [(1r)-2-methoxy-2-oxo-1-phenylethyl]azanium;chloride
12. D-
13. A-phenylglycine Methyl Ester Hydrochloride
14. H-d-phg-ome Hydrochloride
15. Methyl (2r)-amino(phenyl)acetate Hydrochloride
16. Schembl1143309
17. Dthmtbuwtgvefg-ddwiocjrsa-n
18. Akos015846308
19. Akos015903224
20. Cs-w015540
21. Hy-w014824
22. Sy020988
23. (r)-2-phenylglycinemethylesterhydrochloride
24. (r)-2-phenylglycine Methyl Ester Hcl
25. Am20060537
26. P2592
27. E70004
28. En300-657365
29. W-107665
30. (r)-methyl 2-amino-2-phenylacetate, Hydrochloride Salt
31. Methyl (r)-(-)-2-amino-2-phenylacetate Hydrochloride
32. (r)-(-)-2-phenylglycine Methyl Ester Hydrochloride, >=95%
33. (r)-(-)-2-amino-2-phenylacetic Acid Methyl Ester Hydrochloride
34. Benzeneacetic Acid, A-amino-, Methyl Ester, Hydrochloride, (ar)-
| Molecular Weight | 201.65 g/mol |
|---|---|
| Molecular Formula | C9H12ClNO2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 52.3 |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 153 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |







