




1. 179162-55-1
2. Benzoic Acid, 4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]-
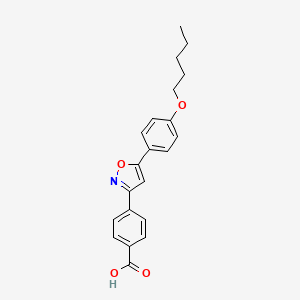
3. 4-[5-(4-pentyloxyphenyl)isoxazol-3-yl]benzoic Acid
4. 4-[5-(4-pentoxyphenyl)-1,2-oxazol-3-yl]benzoic Acid
5. 4-{5-[4-(pentyloxy)phenyl]isoxazol-3-yl}benzoic Acid
6. Micafungin Impurity 3
7. Schembl2353533
8. Mfcd20267356
9. Zinc79047850
10. Akos025395935
11. Ds-8489
12. Sb18167
13. Db-113097
14. Am20090710
15. Cs-0160970
16. F52834
17. A924886
18. A1-01692
19. 4-(5-(4-(pentyloxy)phenyl)isoxazol-3-yl)benzoicacid
20. 4-[5-(4-n-pentyloxyphenyl)isoxazol-3-yl]benzoic Acid
21. 4-[5-(4-pentyloxyphenyl)isoxazole-3-yl]benzoic Acid
22. 4-(5-{4-[(pent-1-yl)oxy]phenyl}isoxazol-3-yl)benzoic Acid
23. 4-{5-[4-(pentyloxy)phenyl]-1,2-oxazol-3-yl}benzoic Acid
24. 4-{5-[4-(pentyloxy)phenyl]-1,2-oxazol-3-yl}benzoic Acid, 3-(4-carboxyphenyl)-5-[4-(pentyloxy)phenyl]-1,2-oxazole
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C21H21NO4 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 351.14705815 g/mol |
| Monoisotopic Mass | 351.14705815 g/mol |
| Topological Polar Surface Area | 72.6 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






