
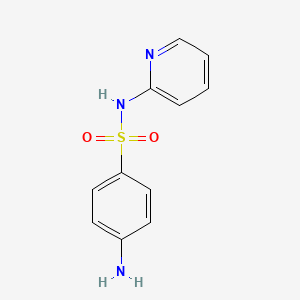

1. Sulphapyridin
2. Sulphapyridine
1. 144-83-2
2. Sulphapyridine
3. 2-sulfapyridine
4. Sulfidin
5. Sulfidine
6. 2-sulfanilamidopyridine
7. 2-sulfanilylaminopyridine
8. Streptosilpyridine
9. Coccoclase
10. Eubasinum
11. Piridazol
12. Pyridazol
13. Eubasin
14. Haptocil
15. Plurazol
16. Relbapiridina
17. Pyriamid
18. Septipulmon
19. Thioseptal
20. Adiplon
21. Dagenan
22. Trianon
23. Ronin
24. 2-sulfanilamidopyridin
25. N-2-pyridylsulfanilamide
26. Solfapiridina
27. Sulfapiridina
28. Sulfapyridinum
29. 4-amino-n-pyridin-2-ylbenzenesulfonamide
30. N1-2-pyridylsulfanilamide
31. N(sup 1)-2-pyridylsulfanilamide
32. 2-(4-aminobenzenesulfonamido)pyridine
33. 2-(p-aminobenzenesulphonamido)pyridine
34. 4-amino-n-pyridin-2-yl-benzenesulfonamide
35. 4-amino-n-(pyridin-2-yl)benzenesulfonamide
36. Benzenesulfonamide, 4-amino-n-2-pyridinyl-
37. 4-(2-pyridinylsulfonyl)aniline
38. 4-[(2-pyridylamino)sulfonyl]aniline
39. N(sup1)-pyridylsulfanilamide
40. M+b 693
41. N(1)-pyridylsulfanilamide
42. N(1)-2-pyridylsulfanilamide
43. A-499
44. Sulfanilamide, N1-2-pyridyl-
45. 4-amino-n,2-pyridinylbenzenesulfonamide
46. 4-amino-n-(pyridin-2-yl)benzene-1-sulfonamide
47. M + B 693
48. Nsc 4753
49. Nsc 41791
50. Sulfanilamide, N(sup 1)-2-pyridyl-
51. 4-amino-n-(2-pyridinyl)benzenesulfonamide
52. Chebi:132842
53. Nsc-4753
54. 4-amino-n-2-pyridinylbenzenesulfonamide
55. Nsc-41791
56. Y5v2n1ke8u
57. Mls000069725
58. Nsc4753
59. 2-sulfanilyl Aminopyridine
60. Ncgc00016408-01
61. Cas-144-83-2
62. Smr000058213
63. Solfapiridina [dcit]
64. Dsstox_cid_6067
65. Dsstox_rid_78003
66. Dsstox_gsid_26067
67. Wln: T6nj Bmswr Dz
68. 4-amino-n-(2-pyridyl)benzenesulfonamide
69. Sulfapyridinum [inn-latin]
70. Sulfapiridina [inn-spanish]
71. 2-sulfanilamidopyridin [german]
72. M And B 693
73. Sulfapyridine (tn)
74. M&b 693
75. 4-amino-n-2-pyridinyl-benzenesulfonamide
76. Sr-01000000207
77. N'-2-pyridylsulfanilide
78. Einecs 205-642-7
79. Sulfapyridine (usp/inn)
80. Unii-y5v2n1ke8u
81. Ai3-01049
82. Sulfapyridine [usp:inn:ban]
83. Sulfapyridine Iii
84. Sfy
85. Albb-006215
86. Prestwick_1015
87. Sulfapyridine-13c6
88. Spectrum_000996
89. Sulfapyridine (dagenan)
90. Prestwick0_000762
91. Prestwick1_000762
92. Prestwick2_000762
93. Prestwick3_000762
94. Spectrum2_001323
95. Spectrum3_001773
96. Spectrum4_000346
97. Spectrum5_001182
98. Sulfapyridine [mi]
99. Chembl700
100. Epitope Id:122235
101. Ec 205-642-7
102. Sulfapyridine [inn]
103. Cid_5336
104. Sulfapyridine, >=99.0%
105. Oprea1_344996
106. Schembl44219
107. Bspbio_000804
108. Bspbio_003265
109. Kbiogr_000751
110. Kbioss_001476
111. Ao-801/41077453
112. Divk1c_000216
113. Spectrum1500551
114. Sulfapyridine [mart.]
115. Spbio_001445
116. Spbio_002743
117. Sulfapyridine [usp-rs]
118. Sulfapyridine [who-dd]
119. Bpbio1_000886
120. Zinc2105
121. Dtxsid3026067
122. Bdbm39340
123. Gechumimrbomgk-uhfffaoysa-
124. Hms500k18
125. Kbio1_000216
126. Kbio2_001476
127. Kbio2_004044
128. Kbio2_006612
129. Kbio3_002766
130. Ninds_000216
131. 4-(2-pyridinylsulfonyl)aniline #
132. Hms1570i06
133. Hms1921c03
134. Hms2092k05
135. Hms2097i06
136. Hms2233h20
137. Hms3372n13
138. Hms3714i06
139. Pharmakon1600-01500551
140. Sulfapyridine [orange Book]
141. Hy-b0212
142. Nsc41791
143. Sulfapyridine [usp Impurity]
144. Tox21_110426
145. Ccg-39261
146. Mfcd00038036
147. Nsc757329
148. S1617
149. Stk292439
150. Sulfapyridine [usp Monograph]
151. 2-(p-aminobenzenesulfonamido)pyridine
152. Akos000121424
153. Tox21_110426_1
154. Cs-2157
155. Db00891
156. Hs-0063
157. M&b-693
158. Nsc-757329
159. Idi1_000216
160. Upcmld0enat5889155:001
161. Ncgc00016408-02
162. Ncgc00016408-03
163. Ncgc00016408-04
164. Ncgc00016408-05
165. Ncgc00016408-06
166. Ncgc00016408-09
167. Ncgc00016408-10
168. Ncgc00022008-03
169. Ncgc00022008-04
170. Sulfanilamide, N1-2(1h)-pyridylidene-
171. Sulfapyridine 100 Microg/ml In Methanol
172. Ac-26818
173. Sbi-0051525.p003
174. Sulfapyridine Melting Point Standard
175. 4-azanyl-n-pyridin-2-yl-benzenesulfonamide
176. Db-042750
177. A 499
178. Ab00052100
179. Bb 0258406
180. Ft-0631839
181. Ft-0674744
182. Sulfasalazine Impurity J [ep Impurity]
183. D02434
184. Ab00052100_14
185. Ab00052100_15
186. A808279
187. Sulfapyridine, Vetranal(tm), Analytical Standard
188. Q3976827
189. Sr-01000000207-2
190. Sr-01000000207-3
191. W-108148
192. Brd-k41406082-001-05-5
193. Brd-k41406082-001-09-7
194. Z271004858
195. 4-amino-n-(1,2-dihydropyridin-2-ylidene)benzenesulfonamide
196. Sulfapyridine, European Pharmacopoeia (ep) Reference Standard
197. 4-amino-n-(pyrimidin-2-yl) Benzenesulfonamide (sulfadiazine) (14)
198. Sulfapyridine, United States Pharmacopeia (usp) Reference Standard
199. Sulfapyridine Melting Point Standard, United States Pharmacopeia (usp) Reference Standard
200. Sulfapyridine Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 249.29 g/mol |
|---|---|
| Molecular Formula | C11H11N3O2S |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 249.05719778 g/mol |
| Monoisotopic Mass | 249.05719778 g/mol |
| Topological Polar Surface Area | 93.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of dermatitis herpetiformis, benign mucous membrane pemphigoid and pyoderma gangrenosum
Sulfapyridine is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EB - Short-acting sulfonamides
J01EB04 - Sulfapyridine
Absorption
Approximately 60-80%
Hepatic.
Sulfapyridine has known human metabolites that include Sulfapyridine, N-acetyl.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
6-14 hours.
Sulfapyridine is a competitive inhibitor of the bacterial enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid by means of processing the substrate para-aminobenzoic acid (PABA). Dihydropteroate synthetase activity is vital in the synthesis of folate, and folate is required for cells to make nucleic acids, such as DNA or RNA. So if DNA molecules cannot be built, the cell cannot divide.
BUILDING BLOCK


