

1. 1195768-06-9
2. Dabrafenib (mesylate)
3. Gsk 2118436b
4. Dabrafenib Mesilate
5. Dabrafenib Mesylate [usan]
6. Unii-b6dc89i63e
7. Gsk-2118436 Mesylate
8. Gsk-2118436b
9. Methane Sulfonate Salt
10. Gsk2118436b
11. Gsk2118436 Mesylate
12. B6dc89i63e
13. Chebi:75048
14. Gsk-2118436b Mesylate
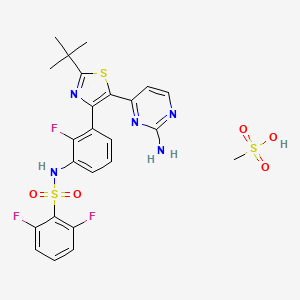
15. N-(3-(5-(2-aminopyrimidin-4-yl)-2-(tert-butyl)thiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide Methanesulfonate
16. Gsk2118436b, Methane Sulfonate Salt
17. Gsk-2118436 Methanesulfonate Salt
18. Gsk-2118436b Methanesulfonate Salt
19. Gsk2118436 Mesylate;gsk 2118436b
20. Dabrafenib Mesilate (jan)
21. Dabrafenib Mesylate (gsk-2118436)
22. Dabrafenib Mesylate (usan)
23. Dabrafenib Mesilate [jan]
24. Taflinar
25. N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide;methanesulfonic Acid
26. Dabrafenib Methanesulfonate
27. Tafinlar (tn)
28. Gsk2118436 Methane Sulfonate Salt
29. Dabrafenib Monomesylate
30. Gsk 2118436 Mesylate
31. Schembl1127269
32. Chembl2105729
33. Dabrafenib Mesylate [mi]
34. Dtxsid70152500
35. Amy30045
36. Bcp04738
37. Ex-a1559
38. Dabrafenib Mesilate [who-dd]
39. Hy-14660a
40. Mfcd20922872
41. S5069
42. Akos025396661
43. Ccg-270246
44. Cs-1641
45. Dabrafenib Mesylate [orange Book]
46. Ac-31302
47. As-17010
48. Ft-0696677
49. D10104
50. A903491
51. Sr-01000941590
52. Sr-01000941590-1
53. Q27145089
54. Gsk2118436 Ms Salt, Dabrafenib Ms Salt, Gsk2118436a Ms Salt
55. Benzenesulfonamide, N-(3-(5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl)- 2-fluorophenyl)-2,6-difluoro-, Methanesulfonate (1:1)
56. Benzenesulfonamide, N-[3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluoro-, Methanesulfonate (1:1)
57. Dabrafenib Mesylaten-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide, Methanesulfonic Acid; Dabrafenib Mesylate
58. N-(3-(5-(2-aminopyrimidin-4-yl)-2-(1,1-dimethylethyl)thiazol-4-yl)-2-fluorophenyl)-2,6- Difluorobenzenesulfonamide Monomethanesulfonate
59. N-{3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide Methanesulfonate
60. N-{3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide Methanesulfonate
| Molecular Weight | 615.7 g/mol |
|---|---|
| Molecular Formula | C24H24F3N5O5S3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 6 |
| Exact Mass | 615.08916689 g/mol |
| Monoisotopic Mass | 615.08916689 g/mol |
| Topological Polar Surface Area | 210 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 910 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 1 | |
|---|---|
| Drug Name | TAFINLAR |
| Active Ingredient | DABRAFENIB MESYLATE |
| Company | NOVARTIS PHARMS CORP (Application Number: N202806. Patents: 7994185, 8415345, 8703781, 8835443, 8952018, 9233956) |
* Melanoma:
Dabrafenib as monotherapy or in combination with trametinib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation (see sections 4. 4 and 5. 1).
* Adjuvant treatment of melanoma:
Dabrafenib in combination with trametinib is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.
* Non-small cell lung cancer (NSCLC):
Dabrafenib in combination with trametinib is indicated for the treatment of adult patients with advanced non-small cell lung cancer with a BRAF V600 mutation.
Treatment of melanoma, Treatment of solid malignant tumours (excluding melanoma)
L01EC02
BUILDING BLOCK


