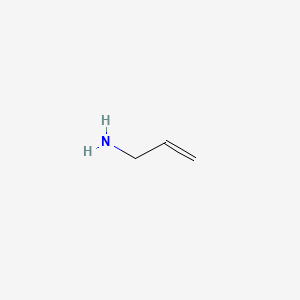

1. 3 Aminopropylene
2. 3-aminopropylene
1. 107-11-9
2. Prop-2-en-1-amine
3. 2-propen-1-amine
4. Monoallylamine
5. 3-aminopropene
6. 3-aminopropylene
7. 2-propenamine
8. 3-amino-1-propene
9. 2-propenylamine
10. Allyl Amine
11. Nsc 7600
12. 30551-89-4
13. 2-propen-1-ylamine
14. Nsc-7600
15. 48g762t011
16. Allylaminehydrochloride
17. Allylamin
18. Ccris 4746
19. Hsdb 2065
20. Einecs 203-463-9
21. Un2334
22. Ailylamine
23. Brn 0635703
24. Allyl-amine
25. N-allylarnine
26. N-allylamine
27. Ai3-23214
28. 2-propenyl Amine
29. Unii-48g762t011
30. (2-propenyl)amine
31. Allylamine, 98%
32. 1-amino-2-propene
33. Allylamine, >=99%
34. Allylamine [mi]
35. Poly(allylamine) Solution
36. Allylamine [hsdb]
37. Ch2=chch2nh2
38. Ec 203-463-9
39. 4-04-00-01057 (beilstein Handbook Reference)
40. Wln: Z2u1
41. Allylamine;3-amino-1-propene
42. Chembl57286
43. Dtxsid8024440
44. Nsc7600
45. Chebi:188938
46. Allylamine [un2334] [poison]
47. Bcp27099
48. Str00193
49. Bdbm50225454
50. Mfcd00008199
51. Stl185583
52. Zinc17654097
53. Akos000119634
54. Allylamine, Purum, >=98.0% (gc)
55. Un 2334
56. Allylamine, Puriss., >=99.5% (gc)
57. Ncgc00159381-02
58. A0219
59. Q417414
60. F2190-0363
| Molecular Weight | 57.09 g/mol |
|---|---|
| Molecular Formula | C3H7N |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 57.057849228 g/mol |
| Monoisotopic Mass | 57.057849228 g/mol |
| Topological Polar Surface Area | 26 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 17.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/Investigators/ studied the uptake, tissue distribution, toxicokinetics, and excretion of allylamine by giving rats (14)C-allylamine (1.5 uCi/kg; 150 mg/kg) by gavage. Rats were killed at intervals up to 2 hr, and multiple tissues were sampled. Aorta showed the highest counts of (14)C-label at most times (5-10-fold higher than most other organs, 100-fold higher than blood), although a minority of aortas had very low counts. Coronary arteries dissected from the hearts showed consistently higher (14)C-label than myocardium. Liver counts, which were high at 5 min, decreased rapidly; kidney counts slowly increased until 45 min, then decreased rapidly, consistent with an excretory function for this organ. Counts of (14)C-label were lower in all other organs, including lung, skeletal muscle, brain, testes, pancreas, adrenal, spleen, fat, and blood. Toxicokinetic study showed a very rapid absorption rate and short half-lives (less than 1 hr) for those organs which reasonably fit a toxicokinetic one-compartment model. (14)C-label was rapidly excreted in the urine; approximately 60% of the dose given was recovered by 24 hr. No counts were found in feces. These studies indicate that allylamine--or its metabolite(s)--has a unique predilection for elastic and muscular arteries, such as aorta and coronary arteries. This relatively specific cardiovascular toxin acts as a highly polar, highly water soluble substance, which is rapidly absorbed from the gastrointestinal tract, has a short half-life in most tissues, and is rapidly excreted in the urine. ...
PMID:4012794 Boor PJ; Toxicology 35 (3): 167-77 (1985)
... Allylamine, a known cardiovascular toxin, is metabolized in vitro to acrolein, /and/ has been hypothesized to act as a distal toxin. In this study, 3-hydroxypropylmercapturic acid was isolated and identified by MS, NMR, and 2D-NMR spectroscopy as the sole urinary metabolite of allylamine metabolism in vivo. Parallel experiments showed reduced glutathione (GSH) depletion in several organs (most marked in aorta, blood, and lung), which is consistent with GSH conjugation of the proposed acrolein intermediate. These findings indicate that allylamine was metabolized in vivo to a highly reactive aldehyde which was converted to a mercapturic acid through a GSH conjugation pathway. ...
PMID:3689456 Boor PJ et al; Biochem Pharmacol 36 (24): 4347-53 (1987)
Acrolein was detected in homogenates of rat aorta, lung, skeletal muscle and heart incubated with allylamine. ... Hydrogen peroxide, a product of oxidative deamination, was generated during allylamine oxidation. Acrolein was also produced by bovine plasma amine oxidase & porcine kidney diamine oxidase but not by rat liver or brain homogenates. ... Allylamine competitively inhibited benzylamine oxidation in rat aorta, but pargyline-sensitive monoamine oxidase was not involved in acrolein production. The high activity in aorta, the competition with benzylamine, and the sensitivity to benzylamine oxidase inhibitors indicate that benzylamine oxidase is the active enzyme in oxidizing allylamine. ...
PMID:7066019 Nelson TJ, Boor PJ; Biochem Pharmacol 31 (4): 509 (1982)
... /Investigators/ tested whether allylamine (AA) or acrolein (1, 10, 100, and 1000 uM), a highly reactive product of AA metabolism by semicarbazide-sensitive amine oxidase (SSAO), could contract coronary artery (CA) or thoracic aorta (TA) in vitro and if the AA effects involved SSAO. AA or acrolein produced a similar pattern of responses in both CA and TA rings at 100 and 1000 uM, including (1) increased basal tension, (2) enhanced agonist-induced contraction (hypercontractility or vasospasm), (3) remarkable, agonist-induced slow wave vasomotion (vasospasm), and (4) irreversible reduction in vessel contractility after 1 mM exposure. Endothelium-dependent acetylcholine-induced relaxation was not altered during vasospasm in either vessel. Pretreatment with the SSAO inhibitor semicarbazide (1 mM; 10 min) prevented or significantly reduced the majority of AA's effects in both CA and TA rings and inhibited 100% of the SSAO activity present in rat TA and human CA and TA. A two-step model for AA induction of CA vasospasm and resultant myocardial necrosis /was proposed/: (1) metabolism of AA to acrolein by coronary arterial SSAO activity and (2) acrolein induction of CA vasospasm independent of endothelial injury-a novel path.
PMID:11543647 Conklin DJ et al; Toxicol Appl Pharmacol 175 (2): 149-59 (2001)
/Investigators/ studied the uptake, tissue distribution, toxicokinetics, and excretion of allylamine by giving rats (14)C-allylamine (1.5 uCi/kg; 150 mg/kg) by gavage. ... Toxicokinetic study showed a very rapid absorption rate and short half-lives (less than 1 hr) for those organs which reasonably fit a toxicokinetic one-compartment model. ...
PMID:4012794 Boor PJ; Toxicology 35 (3): 167-77 (1985)
Aortic smooth muscle cells (SMC) modulate from a contractile to a proliferative phenotype upon subchronic exposure to allylamine. The present studies were designed to determine if this phenotypic modulation is associated with alterations in the metabolism of membrane phosphoinositides. (32)P incorporation into phosphatidylinositol 4-phosphate (PIP), phosphatidylinositol 4,5-bisphosphate (PIP2), and phosphatidic acid (PA) was lower by 31, 35, and 22%, respectively, in SMC from allylamine-treated animals relative to controls. In contrast, incorporation of (3)H-myoinositol into inositol phosphates did not differ in allylamine cells relative to control cells. Exposure to dibutyryl (db) cAMP (0.2 mM) and theophylline (0.1 mM) reduced (32)P incorporation into PIP and PIP2 in SMC from both experimental groups. Under these conditions, a decrease in (3)H-myoinositol incorporation into inositol 1-phosphate was only observed in allylamine cells. The effects of db cAMP and theophylline in allylamine and control SMC correlated with a marked decrease in cellular proliferation. These results suggest that alterations in phosphoinositide synthesis and/or degradation contribute to the enhanced proliferation of SMC induced by allylamine. To further examine this concept, the effects of agents which modulate protein kinase C (PKC) activity were evaluated. Sphingosine (125-500 ng/mL), a PKC inhibitor, decreased SMC proliferation in allylamine, but not control cells. 12-O-Tetradecanoylphorbol-13-acetate (1-100 ng/mL), a PKC agonist, stimulated proliferation in control cells, but inhibited proliferation in cells from allylamine-treated animals. /The authors/ conclude that allylamine-induced phenotypic modulation of SMC is associated with alterations in phosphoinositide metabolism.
PMID:2170166 Cox LR et al; Exp Mol Pathol Aug; 53 (1): 52-63 (1990)
Absorption spectra showed that allylamine formed a complex with pyridoxal phosphate in soln at ph 5.2, 7.4 and 8.0. At 1x10-2 M, allylamine inhibited activity of serum glutamic-oxalacetic transaminase by 22.5%, and at 1x10-4 M it inhibited serum glutamic-pyruvic transaminase by 13.2%. It inhibited activity of serum enzymes in vitro at same concn as isonicotinic acid hydrazide. IV injection of 60 mg/kg into rats decreased activities of hepatic glutamic-oxalacetic transaminase and glutamic-pyruvic transaminase by about 50%, when these levels were determined about 5 min after injection.
Kuzuya F et al; J Nutr 93 (3): 280 (1967)

