16 Oct 2025
// PRESS RELEASE
26 Feb 2025
// PRESS RELEASE
03 Feb 2025
// PRESS RELEASE
 KEY EXCIPIENTS
KEY EXCIPIENTS
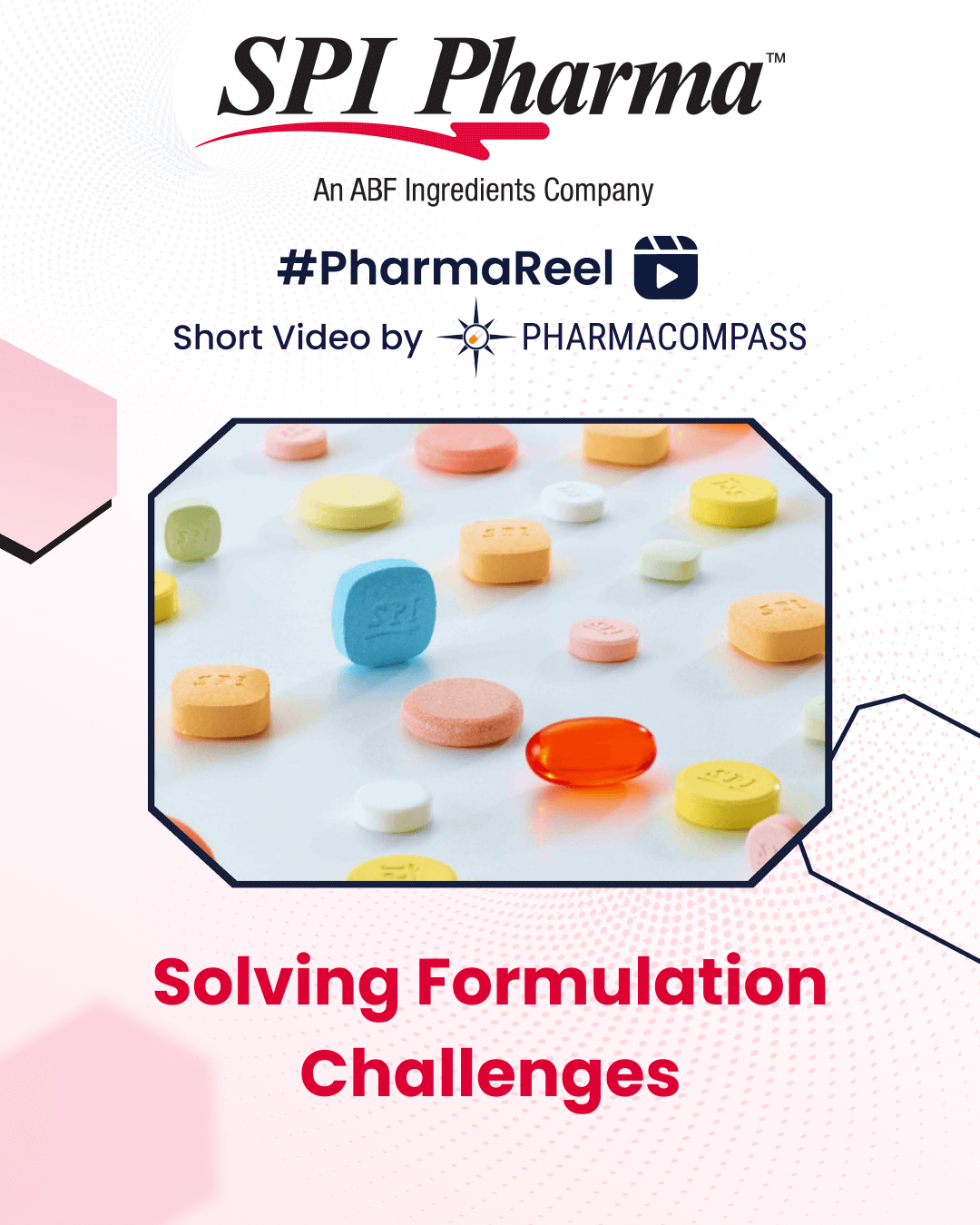
SPI Pharma has been solving formulation challenges using superior functional materials.
About
International Sweetene...International Sweetener Colloquium
Industry Trade Show
Not Confirmed
22-25 February, 2026
Industry Trade Show
Not Confirmed
23-25 February, 2026
Clinical Trial Supply ...Clinical Trial Supply Europe
Industry Trade Show
Not Confirmed
24-25 February, 2026
CONTACT DETAILS




Events
Webinars & Exhibitions

International Sweetene...International Sweetener Colloquium
Industry Trade Show
Not Confirmed
22-25 February, 2026
Industry Trade Show
Not Confirmed
23-25 February, 2026
Clinical Trial Supply ...Clinical Trial Supply Europe
Industry Trade Show
Not Confirmed
24-25 February, 2026
VLOG #PharmaReel
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/excipient-market-overview-evonik-launches-high-purity-excipients-india-mandates-disclosures-from-march-2026
https://www.pharmacompass.com/radio-compass-blog/excipient-market-overview-roquette-announces-restructuring-post-iff-pharma-buyout-who-fda-advance-regulatory-frameworks
https://www.pharmacompass.com/radio-compass-blog/excipient-market-overview-roquette-seqens-evonik-make-strategic-moves-new-guidelines-deal-with-contamination

16 Oct 2025
// PRESS RELEASE
https://www.spipharma.com/en/insights-and-events/news-blog/discover-what-s-next-from-spi-pharma-at-cphi-worldwide/

26 Feb 2025
// PRESS RELEASE
https://www.spipharma.com/en/insights-and-events/news-blog/celebrating-excellence-in-workplace-safety-shane-balcoms-life-saving-actions/

03 Feb 2025
// PRESS RELEASE
https://www.spipharma.com/en/insights-and-events/news-blog/enhancing-patient-experience-with-ultraburst-the-ultimate-odt-solution/

11 Dec 2024
// PRESS RELEASE
https://www.spipharma.com/en/insights-and-events/news-blog/beyond-pharma-spi-pharma-s-impact-across-industries/

17 Jul 2024
// PR NEWSWIRE
https://www.prnewswire.com/news-releases/spi-pharma-inc-and-inimmune-corp-partner-to-develop-and-commercialize-innovative-vaccine-adjuvant-systems-302198093.html

08 Jan 2024
// PRESS RELEASE
https://www.spipharma.com/en/insights-and-events/news-blog/embracing-patient-centric-formulations-for-a-healthier-tomorrow/
Excipients
Excipients by Ingredients
Excipients By applications
REF. STANDARDS & IMPURITIES
ABOUT THIS PAGE
SPI Pharma is a supplier offers 10 products (APIs, Excipients or Intermediates).
Find a price of Magnesium Hydroxide bulk with DMF, CEP offered by SPI Pharma
Find a price of Aluminum bulk with CEP offered by SPI Pharma
Find a price of Calcium Carbonate bulk with DMF offered by SPI Pharma
Find a price of Magnesium Hydroxide bulk with CEP offered by SPI Pharma
Find a price of Aluminium Hydroxide bulk offered by SPI Pharma
Find a price of Calcium Carbonate bulk offered by SPI Pharma
Find a price of Magaldrate bulk offered by SPI Pharma
Find a price of Magnesium Carbonate bulk offered by SPI Pharma
Find a price of Magnesium Hydroxide bulk offered by SPI Pharma
Find a price of Paracetamol bulk offered by SPI Pharma


 SPI Pharma
SPI Pharma