

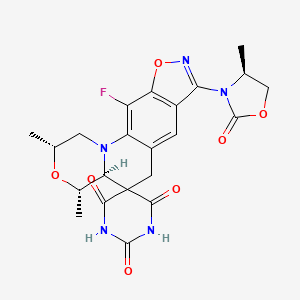

1. (2r,4s,4as)-11-fluoro-2,4-dimethyl-8-((4s)-4-methyl-2-oxo-1,3-oxazolidin-3-yl)-1,2,4,4a-tetrahydro-2'h,6h-spiro((1,2)-oxazolo(4,5-g)(1,4)oxazino(4,3-a)quinoline-5,5'-pyrimidine)-2',4',6'(1'h,3'h)-trione
2. Azd-0914
3. Azd0914
4. Etx-0914
5. Etx0914
1. 1620458-09-4
2. Azd0914
3. Azd-0914
4. Zoliflodacin [usan]
5. Etx0914
6. Etx-0914
7. Fwl2263r77
8. (2r,4s,4as)-11-fluoro-2,4-dimethyl-8-((4s)-4-methyl-2-oxo-1,3-oxazolidin-3-yl)-1,2,4,4a-tetrahydro-2'h,6h-spiro((1,2)-oxazolo(4,5-g)(1,4)oxazino(4,3-a)quinoline-5,5'-pyrimidine)-2',4',6'(1'h,3'h)-trione
9. Unii-fwl2263r77
10. Zoliflodacin(azd0914)
11. Zoliflodacin (usan/inn)
12. Zoliflodacin [inn]
13. Zoliflodacin [who-dd]
14. Azd 0914 - Bio-x
15. Chembl3544978
16. Schembl15879500
17. Gtpl10875
18. Azd0914etx0914
19. Ext0914
20. Bdbm139376
21. Dtxsid101028418
22. Azd 0914
23. Ex-a2620
24. Ext-0914
25. Etx0914; Azd0914; Zoliflodacin
26. Db12817
27. Ac-35757
28. Ba164192
29. Hy-17647
30. Cs-0016917
31. D11726
32. Us8889671, 5
33. Q27278240
34. (2r,4s,4as)-11-fluoro-2,4-dimethyl-8-((s)-4-methyl-2-oxooxazolidin-3-yl)-1,2,4,4a-tetrahydro-2'h,6h-spiro[isoxazolo[4,5-g][1,4]oxazino[4,3-a]quinoline-5,5'-pyrimidine]-2',4',6'(1'h,3'h)-trione
35. (2r,4s,4as)-11-fluoro-2,4-dimethyl-8-((s)-4-methyl-2-oxooxazolidin-3-yl)-2,4,4a,6-tetrahydro-1h,1'h-spiro[isoxazolo[4,5-g][1,4]oxazino[4,3-a]quinoline-5,5'-pyrimidine]-2',4',6'(3'h)-trione
36. (4'r,6's,7's)-17'-fluoro-4',6'-dimethyl-13'-[(4s)-4-methyl-2-oxo-1,3-oxazolidin-3-yl]spiro[1,3-diazinane-5,8'-5,15-dioxa-2,14-diazatetracyclo[8.7.0.02,7.012,16]heptadeca-1(17),10,12(16),13-tetraene]-2,4,6-trione
37. (7s,11r,13s)-3-((4s)-2-oxo-4-methyltetrahydrooxazole-3-yl)-9-fluoro-11,13-dimethyl-8,7-(ethanoxymethano)-7,8,1',2',3',4'-hexahydrospiro[isoxazolo[4,5-g]quinoline-6(5h),5'(6'h)-pyrimidine]-2',4',6'-trione
38. Spiro(isoxazolo(4,5-g)(1,4)oxazino(4,3-a)quinoline-5(6h),5'(2'h)-pyrimidine)-2',4',6'(1'h,3'h)-trione, 11-fluoro-1,2,4,4a-tetrahydro-2,4-dimethyl-8-((4s)-4-methyl-2-oxo-3-oxazolidinyl)-, (2r,4s,4as)-
| Molecular Weight | 487.4 g/mol |
|---|---|
| Molecular Formula | C22H22FN5O7 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 1 |
| Exact Mass | 487.15032622 g/mol |
| Monoisotopic Mass | 487.15032622 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 962 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of gonococcal infection
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
