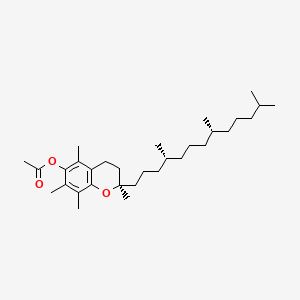

1. 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-1-benzopyran-6-ol
2. Acetate, Tocopherol
3. Alpha Tocopherol
4. Alpha Tocopherol Acetate
5. Alpha Tocopherol Hemisuccinate
6. Alpha Tocopherol Succinate
7. Alpha Tocopheryl Calcium Succinate
8. Alpha-tocopherol
9. Alpha-tocopherol Hemisuccinate
10. Alpha-tocopherol Succinate
11. Alpha-tocopheryl Calcium Succinate
12. D Alpha Tocopherol
13. D Alpha Tocopheryl Acetate
14. D-alpha Tocopherol
15. D-alpha-tocopheryl Acetate
16. R,r,r-alpha-tocopherol
17. Tocopherol Acetate
18. Tocopherol Succinate
19. Tocopherol, D-alpha
20. Tocopheryl Acetate
21. Vitamin E Succinate
1. Vitamin E Acetate
2. 58-95-7
3. Tocopherol Acetate
4. D-alpha-tocopheryl Acetate
5. Alfacol
6. D-alpha-tocopherol Acetate
7. Ecofrol
8. Contopheron
9. Tofaxin
10. Econ
11. Ephynal Acetate
12. Tokoferol Acetate
13. Tocopheryl Acetate
14. (+)-alpha-tocopherol Acetate
15. Tocopherex
16. Evipherol
17. Tocophrin
18. Erevit
19. Gevex
20. Juvela
21. Combinal E
22. Epsilan-m
23. E-toplex
24. E-ferol
25. Endo E Dompe
26. Alpha-tocopheryl Acetate
27. Spondyvit
28. Copherol 1250
29. Covitol 1100
30. Covitol 1360
31. Vitamin Ealpha Acetate
32. Dl-alpha-tocopheryl Acetate
33. Vitamin E Acetate, D-
34. Nanotopes
35. Simmyungsaengmosu
36. Ephynal
37. Fertilvit
38. Natac
39. Tinoderm E
40. (+)-alpha-tocopheryl Acetate
41. Natur-e Granulate
42. Lutavit E 50
43. 52225-20-4
44. Ccris 4389
45. Alpha-tocopherol Acetate, All Rac
46. Dl-alpha-tocopherylacetate
47. (r,r,r)-alpha-tocopheryl Acetate
48. Einecs 200-405-4
49. Einecs 231-710-0
50. Mfcd00072052
51. D-.alpha.-tocopherol Acetate
52. Tocopheryl Acetate, D-alpha-
53. Vitamin E Acetate (d-form)
54. Unii-9e8x80d2l0
55. Unii-a7e6112e4n
56. Vectan (tn)
57. Brn 0097512
58. (2r,4'r,8'r)-alpha-tocopheryl Acetate
59. 54-22-8
60. Chebi:32321
61. A7e6112e4n
62. Alpha-tocopherol Acetate, (2r,4'r,8'r)-
63. D-
64. A-tocopherol Acetate
65. D-alpha Tocoferil Acetate
66. Tocopherolacetate, Alpha-
67. Dl-alpha Tocopheryl Acetate
68. Mfcd00072042
69. Tocopherol Acetate [jan]
70. Dsstox_cid_1356
71. .alpha.-tocopheryl Acetate
72. D-alpha Tocopheryl Acetate
73. Dsstox_rid_76104
74. Dsstox_rid_78863
75. Tocopherol Acetate (jp17)
76. Chembl1047
77. Rrr-alpha-tocopheryl Acetate
78. (+)-alfa-tocopherol Acetate
79. Dsstox_gsid_21356
80. Dsstox_gsid_31096
81. Ncgc00166253-02
82. Schembl22298
83. Alpha-tocopheryl Acetate, D-
84. (+-)-alpha-tocopherol Acetate
85. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate
86. 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-benzopyran-6-yl Acetate
87. Mls001335985
88. Mls001335986
89. [(2r)-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-yl] Acetate
90. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate, [2r-[2r*(4r*,8r*)]]-
91. (+)-.alpha.-tocopherol Acetate
92. (+)-.alpha.-tocopheryl Acetate
93. .alpha.-tocopherol Acetate
94. Dl-alpha-tocopherylacetate (vitamin E Acetate)
95. Ec 231-710-0
96. T-3376
97. 9e8x80d2l0
98. Hms2230c20
99. Vitamin E Acetate Oil - Synthetic
100. 5-17-04-00169 (beilstein Handbook Reference)
101. Tox21_111491
102. Tox21_111564
103. Tox21_113467
104. Tox21_303444
105. Zinc4172337
106. 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-6-cromanyl Acetate, (+)-
107. 6-cromanol, 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate, (+)-
108. D-vitamin E Acetate
109. (r,r,r)-.alpha.-tocopheryl Acetate
110. 6-chromanol, 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate, (+)-
111. Ccris 6054
112. Akos025117621
113. Tox21_113467_1
114. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate, (2r-(2*(4r*,8r*)))-
115. 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-1-benzopyran-6-yl Acetate, (2r-(2*(4r*,8r*)))-
116. Cas-58-95-7
117. (+-)-alpha-tocopherol Acetateacid Ester
118. Ncgc00095255-08
119. Ncgc00166253-01
120. Ncgc00257504-01
121. As-13784
122. Smr000857327
123. Cas-52225-20-4
124. Einecs 257-757-7
125. O-acetyl-alpha-tocopherol
126. (2r,4'r,8'r)-.alpha.-tocopheryl Acetate
127. Dl-alpha-tocopherol Acetate, >=96% (hplc)
128. 6-chromanol, 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate
129. C13202
130. D01735
131. Dl-alpha-tocopherol Acetate, Analytical Standard
132. [(2r)-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]chroman-6-yl] Acetate
133. 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-b- Enzopyran-6-ol, Acetate
134. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-((4r,8r)-4,8,12-trimethyltridecyl)-, 6-acetate, (2r)-
135. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-((4r,8r)-4,8,12-trimethyltridecyl)-, Acetate, (2r)-
136. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-((4r,8r)-4,8,12-trimethyltridecyl)-, Acetate, (2r)-rel-
137. D-.alpha.-tocopheryl Acetate
138. Q-201933
139. W-109259
140. Eca8c22f-b5d3-4b88-a9b7-af6c600001bb
141. C31h52o3
142. Dl-alpha-tocopherol Acetate, Tested According To Ph.eur.
143. (+)-alpha-tocopherol Acetate, Oil Or Semi-solid, ~1360 Iu/g, Semisynthetic
144. Alpha Tocopheryl Acetate, United States Pharmacopeia (usp) Reference Standard
145. Alpha-tocopherol Acetate, European Pharmacopoeia (ep) Reference Standard
146. Dl-alpha-tocopherol Acetate, Certified Reference Material, Tracecert(r)
147. Dl-alpha-tocopherylacetate (vitamin E Acetate) 10 Microg/ml In Acetonitrile
148. (+)-alpha-tocopherol Acetate, Bioreagent, Suitable For Insect Cell Culture, ~1360 Iu/g
149. (r)-2,5,7,8-tetramethyl-2-((4r,8r)-4,8,12-trimethyltridecyl)chroman-6-yl Acetate
150. 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2h-chromen-6-yl Acetate #
151. Tocopheryl Acetate, A, Pharmaceutical Secondary Standard; Certified Reference Material
152. (2r)-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-3,4-dihydro-2h-chromen-6-yl Acetate
153. [2r-[2r*(4r,8r*)]]-3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-1-benzopyran-6-ol Acetate
154. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, 6-acetate
155. All-rac-alpha-tocopheryl Acetate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
156. Alpha Tocopheryl Acetate
157. Copherol 12250
158. D-
159. Atocopheryl Acetate
160. O-acetyl-
161. A-tocopherol
162. 1406-70-8
163. Alpha-tocopherylis Acetas
164. Ac1l3bmh
165. Vitamin E Acetate, (2r-(2r*(4r*,8r*)))-isomer
166. Dl-
167. A-tocopheryl Acetate
168. Vitamin E Acetate, ((2r*(4r*,8r*))-(+-))-isomer
169. Ac1q1pb2
170. (+)-
171. A-tocopherol Acetate
172. (+)-
173. A-tocopheryl Acetate
174. All-rac-
175. A-tocopheryl Acetate
176. Dl-alpha-tocopherolacetate
177. Nsc-755840
178. (r,r,r)-
179. A-tocopheryl Acetate
180. (2r*(4r*,8r*))-(1)-3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-benzopyran-6-yl Acetate
181. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate,(2r*(4r*,8r*))-(+-)-
182. Ls-245
183. Ft-0624407
184. .alpha.-tocopherol Acetate, D-
185. (2r,4'r,8'r)-
186. A-tocopherol Acetate
187. (2r,4'r,8'r)-
188. A-tocopheryl Acetate
189. Ls-39402
190. Ls-53371
191. Sc-16401
192. Sc-18242
193. Dl-alpha-tocopherol Acetate, 50% Powder Form
194. J10308
195. Dl-alpha-tocopherol Acetate, Ep/usp/fcc Grade
196. Tocopheryl Acetate, D-alpha
197. Vitamin E Acetate (unlabeled)
198. Dtxsid1031096
199. Dtxsid3021356
200. (+)- Alpha -tocopherol Acetate
201. D-alpha-tocopheryl Acetate, 97%
202. Molport-003-928-528
203. Dl-alpha-tocopheryl Acetate, 98%
204. Hy-b1278
205. Tocopheryl Acetate [who-dd]
206. Alpha-tocopherol Acetate, D-
207. Alpha-tocopherol Acetate. D-
208. S3681
209. 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol Acetate
210. Ccg-269474
211. Db14002
212. Nsc 755840
213. .alpha.-tocopherol Acetate. D-
214. .alpha.-tocopherol Acetate [mi]
215. Tocopheryl Acetate, .alpha., D-
216. (2r)-3,4-dihydro-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-2h-1-benzopyran-6-ol 6-acetate
217. [2r*(4r*,8r*)]-()-3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2h-benzopyran-6-yl Acetate
218. 133-80-2
219. 2h-1-benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, Acetate,
220. 2h-1-benzopyran-6-ol,3,4-dihydro-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-,6-acetate, (2r)-
221. Ak176402
222. All-rac-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2h-1-benzopyran-6-yl Acetate
223. J24.807j
224. D-alpha Tocoferil Acetate [mart.]
225. Rrr-alpha-tocopheryl Acetate [fcc]
226. Tocopheryl Acetate,d-alpha [vandf]
227. Cs-0013056
228. T2322
229. A11606
230. D70796
231. Rrr-alpha-tocopheryl Acetate Concentrate
232. Tocopheryl Acetate (vitamin E Acetate) Solution
233. A865381
234. Q364160
235. Rrr-alpha-tocopheryl Acetate Concentrate [fcc]
236. Unii-wr1wpi7ew8 Component Zakowwreflajot-cefnrusxsa-n
237. Dl-alpha-tocopherylacetate (vitamin E Acetate) 100 Microg/ml In Methanol
238. Vitamin E Acetate (dimethyl-13c2, Acetyl-13c2, 99%; Dimethyl-d6, 98%)
239. (2r)-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-3,4-dihydro-2h-1-benzopyran-6-yl Acetate
240. 12741-00-3
241. 1407-18-7
242. 26243-95-8
243. 2h-1-benzopyran-6-ol,3,4-dihydro-2,5,7,8-tetramethyl-2-[(4r,8r)-4,8,12-trimethyltridecyl]-,acetate, (2r)-
| Molecular Weight | 472.7 g/mol |
|---|---|
| Molecular Formula | C31H52O3 |
| XLogP3 | 10.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 14 |
| Exact Mass | 472.39164552 g/mol |
| Monoisotopic Mass | 472.39164552 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 602 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._ Vitamin E, known for its antioxidant activities, is protective against cardiovascular disease and some forms of cancer and has also demonstrated immune-enhancing effects. It may be of limited benefit in some with asthma and rheumatoid arthritis. It may be helpful in some neurological diseases including Alzheimer's, some eye disorders including cataracts, and diabetes and premenstrual syndrome. It may also help protect skin from ultraviolet irradiation although claims that it reverses skin aging, enhances male fertility and exercise performance are poorly supported. It may help relieve some muscle cramps.
The primary health-related use for which alpha-tocopherol acetate is formally indicated is as a dietary supplement for patients who demonstrate a genuine vitamin E deficiency. At the same time, vitamin E deficiency is generally quite rare but may occur in premature babies of very low birth weight (< 1500 grams), individuals with fat-malabsorption disorders (as fat is required for the digestive tract to absorb vitamin E), or individuals with abetalipoproteinemia - a rare, inherited disorder that causes poor absorption of dietary fat - who require extremely large doses of supplemental vitamin E daily (around 100 mg/kg or 5-10 g/day). In all such cases, alpha-tocopherol is largely the preferred form of vitamin E to be administered. Elsewhere, vitamin E's chemical profile as a fat-soluble antioxidant that is capable of neutralizing free radicals in the body continues to generate ongoing interest and study regarding how and whether or not the vitamin can help prevent or delay various chronic diseases associated with free radicals or other potential biological effects that vitamin E possesses like cardiovascular diseases, diabetes, ocular conditions, immune illnesses, cancer, and more. None of these ongoing studies have yet to elucidate any formally significant evidence, however.
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._ Vitamin E has antioxidant activity. It may also have anti-atherogenic, antithrombotic, anticoagulant, neuroprotective, antiviral, immunomodulatory, cell membrane-stabilizing and antiproliferative actions. Vitamin E is a collective term used to describe eight separate forms, the best-known form being alpha-tocopherol. Vitamin E is a fat-soluble vitamin and is an important antioxidant. It acts to protect cells against the effects of free radicals, which are potentially damaging by-products of the body's metabolism. Vitamin E is often used in skin creams and lotions because it is believed to play a role in encouraging skin healing and reducing scarring after injuries such as burns. There are three specific situations when a vitamin E deficiency is likely to occur. It is seen in persons who cannot absorb dietary fat, has been found in premature, very low birth weight infants (birth weights less than 1500 grams, or 3½ pounds), and is seen in individuals with rare disorders of fat metabolism. A vitamin E deficiency is usually characterized by neurological problems due to poor nerve conduction. Symptoms may include infertility, neuromuscular impairment, menstrual problems, miscarriage and uterine degradation. Preliminary research has led to a widely held belief that vitamin E may help prevent or delay coronary heart disease. Antioxidants such as vitamin E help protect against the damaging effects of free radicals, which may contribute to the development of chronic diseases such as cancer. It also protects other fat-soluble vitamins (A and B group vitamins) from destruction by oxygen. Low levels of vitamin E have been linked to increased incidence of breast and colon cancer.
Of the eight separate variants of vitamin E, alpha-tocopherol is the predominant form of vitamin E in human and animal tissues, and it has the highest bioavailability. This is because the liver preferentially resecretes only alpha-tocopherol by way of the hepatic alpha-tocopherol transfer protein (alpha-TTP); the liver metabolizes and excretes all the other vitamin E variants, which is why blood and cellular concentrations of other forms of vitamin E other than alpha-tocopherol are ultimately lower. Furthermore, the term alpha-tocopherol generally refers to a group of eight possible stereoisomers which is often called all-rac-tocopherol for being a racemic mixture of all eight stereoisomers. Of the eight stereoisomers, the RRR-alpha-tocopherol - or sometimes referred to as the d-alpha-tocopherol - stereoisomer is the naturally occurring form of alpha-tocopherol that is perhaps best recognized by the alpha-TTP and has been reported to demonstrate approximately twice the systemic availability of all-rac-tocopherol. As a result, often times (but certainly not always) the discussion of vitamin E - at least within the context of using the vitamin for health-related indications - is generally in reference to the use of RRR- or d-alpha-tocopherol.
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
Absorption
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._ 50 to 80% absorbed from gastrointestinal tract.
Route of Elimination
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._
Volume of Distribution
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._
Clearance
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._
Absorption
When vitamin E is ingested, intestinal absorption plays a principal role in limiting its bioavailability. It is known that vitamin E is a fat-soluble vitamin that follows the intestinal absorption, hepatic metabolism, and cellular uptake processes of other lipophilic molecules and lipids. The intestinal absorption of vitamin E consequently requires the presence of lipid-rich foods. In particular, stable alpha-tocopherol acetate undergoes hydrolysis by bile acid-dependant lipase in the pancreas or by an intestinal mucosal esterase. Subsequent absorption in the duodenum occurs by way of transfer from emulsion fat globules to water-soluble multi- and unilamellar vesicles and mixed micelles made up of phospholipids and bile acids. As the uptake of vitamin E into enterocytes is less efficient compared to other types of lipids, this could potentially explain the relatively low bioavailability of vitamin E. Alpha-tocopherol acetate itself is embedded in matrices where its hydrolysis and its uptake by intestinal cells are markedly less efficient than in mixed micelles. Subsequently, the intestinal cellular uptake of vitamin E from mixed micelles follows in principle two different pathways across enterocytes: (a) via passive diffusion, and (b) via receptor-mediated transport with various cellular transports like scavenger receptor class B type 1, Niemann-Pick C1-like protein, ATP-binding cassette (ABC) transporters ABCG5/ABCG8, or ABCA1, among others. Vitamin E absorption from the intestinal lumen is dependent upon biliary and pancreatic secretions, micelle formation, uptake into enterocytes, and chylomicron secretion. Defects at any step can lead to impaired absorption.. Chylomicron secretion is required for vitamin E absorption and is a particularly important factor for efficient absorption. All of the various vitamin E forms show similar apparent efficiencies of intestinal absorption and subsequent secretion in chylomicrons. During chylomicron catabolism, some vitamin E is distributed to all the circulating lipoproteins. Chylomicron remnants, containing newly absorbed vitamin E, are then taken up by the liver. Vitamin E is secreted from the liver in very low density lipoproteins (VLDLs). Plasma vitamin E concentrations depend upon the secretion of vitamin E from the liver, and only one form of vitamin E, alpha-tocopherol, is ever preferentially resecreted by the liver. The liver is consequently responsible for discriminating between tocopherols and the preferential plasma enrichment with alpha-tocopherol. In the liver, the alpha-tocopherol transfer protein (alpha-TTP) likely is in charge of the discriminatory function, where RRR- or d-alpha-tocopherol possesses the greatest affinity for alpha-TTP. It is nevertheless believed that only a small amount of administered vitamin E is actually absorbed. In two individuals with gastric carcinoma and lymphatic leukemia, the respective fractional absorption in the lymphatics was only 21 and 29 percent of label from meals containing alpha-tocopherol and alpha-tocopheryl acetate, respectively. Additionally, after feeding three separate single doses of 125 mg, 250 mg, and 500 mg to a group of healthy males, the observed plasma peak concentrations (ng/mL) were 1822 +/- 48.24, 1931.00 +/- 92.54, and 2188 +/- 147.61, respectively.
Route of Elimination
The major route of excretion of ingested vitamin E is fecal elimination because of its relatively low intestinal absorption. Excess alpha-tocopherol, as well as forms of vitamin E not preferentially used, are probably excreted unchanged in bile.
Volume of Distribution
When three particular doses alpha-tocopherol were administered to healthy male subjects, the apparent volumes of distribution (ml) observed were: (a) at a single administered dose of 125 mg, the Vd/f was 0.070 +/- 0.002, (b) at dose 250. mg, the Vd/f was 0.127 +/- 0.004, and (c) at dose 500 mg, the Vd/f was 0.232 +/- 0.010.
Clearance
When three specific doses of 125 mg, 250 mg, and 500 mg of alpha-tocopherol were administered as single doses to a group of healthy males, the resultant times of clearance observed, respectively, were: 0.017 +/- 0.015 l/h, 0.011 +/- 0.001 l/h, and 0.019 +/- 0.001 l/h.
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._ Hepatic.
Primary hepatic metabolism of alpha-tocopherol begins in the endoplasmic reticulum with CYP4F2/CYP3A4 dependent -hydroxylation of the aliphatic side-chain, which forms the 13-hydroxychromanol (13-OH) metabolite. Next, peroxisome -oxidation results in 13-carboxychromanol (13-COOH). Following these two steps are five consecutive -oxidation reactions which serve to shorten the alpha-tocopherol metabolite side-chains. The first of these -oxidations occurs still in the peroxisome environment, generating carboxydimethyldecylhydroxychromanol (CDMDHC, 11-COOH). Then, in the mitochondrion, the second -oxidation step forms the carboxymethyloctylhydroxychromanol (CDMOHC, 9-COOH) metabolite. Since both CDMDHC and CDMOHC possess a side-chain length of between 13 to 9 carbon units, they are considered long-chain metabolites. The hydrophobicity of these long-chain metabolites means they are not excreted in the urine but have been found in human microsomes, serum, and feces. The next two -oxidation reactions, still within the mitochondrion environment, produce two intermediate chain metabolites: carboxymethylhexylhydroxychromanol (CDMHHC, 7-COOH), followed by carboxymethylbutylhydroxychromanol (CMBHC, 5-COOH). Both of these intermediate chain metabolites are found in human plasma, feces, and urine. Finally, the last mitochrondrion -oxidation generates the catabolic end product of alpha-tocopherol metabolism: carboxyethyl-hydroxychromans (CEHC, 3'-COOH), which is considered a short-chain metabolite. CEHC has been observed in human plasma, serum, urine, and feces.
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._
The apparent half-life of RRR- or d-alpha-tocopherol in normal subjects is approximately 48 hours.
_In addition to any following information, owing to d-alpha-Tocopherol acetate's closely related chemical nature with alpha-Tocopherol acetate, please also refer to the drug information page for alpha-Tocopherol acetate for further data._ Although all forms of Vitamin E exhibit antioxidant activity, it is known that the antioxidant activity of vitamin E is not sufficient to explain the vitamin's biological activity.
Vitamin E's anti-atherogenic activity involves the inhibition of the oxidation of LDL and the accumulation of oxLDL in the arterial wall. It also appears to reduce oxLDL-induced apoptosis in human endothelial cells. Oxidation of LDL is a key early step in atherogenesis as it triggers a number of events which lead to the formation of atherosclerotic plaque. In addition, vitamin E inhibits protein kinase C (PKC) activity. PKC plays a role in smooth muscle cell proliferation, and, thus, the inhibition of PKC results in inhibition of smooth muscle cell proliferation, which is involved in atherogenesis.
Vitamin E's antithrombotic and anticoagulant activities involves the downregulation of the expression of intracellular cell adhesion molecule(ICAM)-1 and vascular cell adhesion molecule(VCAM)-1 which lowers the adhesion of blood components to the endothelium. In addition, vitamin E upregulates the expression of cytosolic phospholipase A2 and cyclooxygenase (COX)-1 which in turn enhances the release of prostacyclin. Prostacyclin is a vasodilating factor and inhibitor of platelet aggregation and platelet release. It is also known that platelet aggregation is mediated by a mechanism involving the binding of fibrinogen to the glycoprotein IIb/IIIa (GPIIb/IIIa) complex of platelets. GPIIb/IIIa is the major membrane receptor protein that is key to the role of the platelet aggregation response. GPIIb is the alpha-subunit of this platelet membrane protein. Alpha-tocopherol downregulates GPIIb promoter activity which results in reduction of GPIIb protein expression and decreased platelet aggregation. Vitamin E has also been found in culture to decrease plasma production of thrombin, a protein which binds to platelets and induces aggregation. A metabolite of vitamin E called vitamin E quinone or alpha-tocopheryl quinone (TQ) is a potent anticoagulant. This metabolite inhibits vitamin K-dependent carboxylase, which is a major enzyme in the coagulation cascade.
The neuroprotective effects of vitamin E are explained by its antioxidant effects. Many disorders of the nervous system are caused by oxidative stress. Vitamin E protects against this stress, thereby protecting the nervouse system.
The immunomodulatory effects of Vitamin E have been demonstrated in vitro, where alpha-tocopherol increases mitogenic response of T lymphocytes from aged mice. The mechanism of this response by vitamin E is not well understood, however it has been suggested that vitamin E itself may have mitogenic activity independent of its antioxidant activity.
Lastly, the mechanism of action of vitamin E's antiviral effects (primarily against HIV-1) involves its antioxidant activity. Vitamin E reduces oxidative stress, which is thought to contribute to HIV-1 pathogenesis, as well as to the pathogenesis of other viral infections. Vitamin E also affects membrane integrity and fluidity and, since HIV-1 is a membraned virus, altering membrane fluidity of HIV-1 may interfere with its ability to bind to cell-receptor sites, thus decreasing its infectivity.
Vitamin E's antioxidant capabilities are perhaps the primary biological action associated with alpha-tocopherol. In general, antioxidants protect cells from the damaging effects of free radicals, which are molecules that consist of an unshared electron. These unshared electrons are highly energetic and react rapidly with oxygen to form reactive oxygen species (ROS). In doing so, free radicals are capable of damaging cells, which may facilitate their contribution to the development of various diseases. Moreover, the human body naturally forms ROS when it converts food into energy and is also exposed to environmental free radicals contained in cigarette smoke, air pollution, or ultraviolet radiation from the sun. It is believed that perhaps vitamin E antioxidants might be able to protect body cells from the damaging effects of such frequent free radical and ROS exposure. Specifically, vitamin E is a chain-breaking antioxidant that prevents the propagation of free radical reactions. The vitamin E molecule is specifically a peroxyl radical scavenger and especially protects polyunsaturated fatty acids within endogenous cell membrane phospholipids and plasma lipoproteins. Peroxyl free radicals react with vitamin E a thousand times more rapidly than they do with the aforementioned polyunsaturated fatty acids. Furthermore, the phenolic hydroxyl group of tocopherol reacts with an organic peroxyl radical to form an organic hydroperoxide and tocopheroxyl radical. This tocopheroxyl radical can then undergo various possible reactions: it could (a) be reduced by other antioxidants to tocopherol, (b) react with another tocopheroxyl radical to form non-reactive products like tocopherol dimers, (c) undergo further oxidation to tocopheryl quinone, or (d) even act as a prooxidant and oxidize other lipids. In addition to the antioxidant actions of vitamin E, there have been a number of studies that report various other specific molecular functions associated with vitamin E. For example, alpha-tocopherol is capable of inhibiting protein kinase C activity, which is involved in cell proliferation and differentiation in smooth muscle cells, human platelets, and monocytes. In particular, protein kinase C inhibition by alpha-tocopherol is partially attributable to its attenuating effect on the generation of membrane-derived dialglycerol, a lipid that facilitates protein kinase C translocation, thereby increasing its activity. In addition, vitamin E enrichment of endothelial cells downregulates the expression of intercellular cell adhesion molecule (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), thereby decreasing the adhesion of blood cell components to the endothelium. Vitamin E also upregulates the expression of cytosolic phospholipase A2 and cyclooxygenase-1. The increased expression of these two rate-limiting enzymes in the arachidonic acid cascade explains the observation that vitamin E, in a dose-dependent fashion, enhanced the release of prostacyclin, a potent vasodilator and inhibitor of platelet aggregation in humans. Furthermore, vitamin E can inhibit platelet adhesion, aggregation, and platelet release reactions. The vitamin can also evidently inhibit the plasma generation of thrombin, a potent endogenous hormone that binds to platelet receptors and induces aggregation of platelets. Moreover, vitamin E may also be able to decrease monocyte adhesion to the endothellium by downregulating expression of adhesion molecules and decreasing monocyte superoxide production. Given these proposed biological activities of vitamin E, the substance continues to generate ongoing interest and studies in whether or not vitamin E can assist in delaying or preventing various diseases with any one or more of its biologic actions. For instance, studies continue to see whether vitamin E's ability to inhibit low-density lipoprotein oxidation can aid in preventing the development of cardiovascular disease or atherogenesis. Similarly, it is also believed that if vitamin E can decrease the chance of cardiovascular disease then it can also decrease the chance of related diabetic disease and complications. In much the same way, it is also believed that perhaps the antioxidant abilities of vitamin E can neutralize free radicals that are constantly reacting and damaging cellular DNA. Furthermore, it is also believed that free radical damage does contribute to protein damage in the ocular lens - another free radical-mediated condition that may potentially be prevented by vitamin E use. Where it is also suggested that various central nervous system disorders like Parkinson's disease, Alzheimer's disease, Down's syndrome, and Tardive Dyskinesia possess some form of oxidative stress component, it is also proposed that perhaps vitamin E use could assist with its antioxidant action. There have also been studies that report the possibility of vitamin E supplementation can improve or reverse the natural decline in cellular immune function in healthy, elderly individuals. As of this time however, there is either only insufficient data or even contradicting data (where certain doses of vitamin E supplementation could even potentially increase all-cause mortality) on which to suggest the use of vitamin E could formally benefit in any of these proposed indications.