

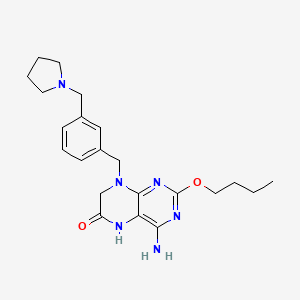

1. 4-amino-2-butoxy-8-(3-(1-pyrrolidinylmethyl)benzyl)-7,8-dihydro-6(5h)-pteridinone
2. Gs-9620
1. Gs-9620
2. 1228585-88-3
3. Gs9620
4. Vesatolimod [inn]
5. 4-amino-2-butoxy-8-(3-(pyrrolidin-1-ylmethyl)benzyl)-7,8-dihydropteridin-6(5h)-one
6. 8-(3-(pyrrolidin-1-ylmethyl)benzyl)-4-amino-2-butoxy-7,8-dihydropteridin-6(5h)-one
7. Gs 9620
8. Chembl2424780
9. O8m467c50g
10. Mfcd25372045
11. 4-amino-2-butoxy-8-[[3-(pyrrolidin-1-ylmethyl)phenyl]methyl]-5,7-dihydropteridin-6-one
12. 6(5h)-pteridinone, 4-amino-2-butoxy-7,8-dihydro-8-((3-(1-pyrrolidinylmethyl)phenyl)methyl)-
13. 4-azanyl-2-butoxy-8-[[3-(pyrrolidin-1-ylmethyl)phenyl]methyl]-5,7-dihydropteridin-6-one
14. Unii-o8m467c50g
15. Vesatolimod (usan/inn)
16. Vesatolimod [usan:inn]
17. Vesatolimod [usan]
18. Vesatolimod [who-dd]
19. Schembl10083191
20. Dtxsid40153741
21. Hms3750i03
22. Amy41043
23. Bcp08305
24. Ex-a2065
25. Bdbm50440284
26. S7221
27. Zinc95616590
28. Akos025290740
29. Ccg-268776
30. Cs-1352
31. Db12687
32. Sb16757
33. Ncgc00390283-01
34. Ncgc00390283-02
35. Ncgc00390283-03
36. Ac-30275
37. As-74316
38. Da-33595
39. Hy-15601
40. Sy028554
41. Ft-0721305
42. D11003
43. Q27285487
44. 4-amino-2-butoxy-7,8-dihydro-8-[[3-(1-pyrrolidinylmethyl)phenyl]methyl]-6(5h)-pteridinone
45. 4-amino-2-butoxy-8-(3-(1-pyrrolidinylmethyl)benzyl)-7,8-dihydro-6(5h)-pteridinone
46. 4-amino-2-butoxy-8-((3-((pyrrolidin-1-yl)methyl)phenyl)methyl)-7,8-dihydropteridin-6(5h)-one
47. 4-amino-2-butoxy-8-({3-[(pyrrolidin-1-yl)methyl]phenyl}methyl)-5,6,7,8-tetrahydropteridin-6-one
48. 9jr
| Molecular Weight | 410.5 g/mol |
|---|---|
| Molecular Formula | C22H30N6O2 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 410.24302422 g/mol |
| Monoisotopic Mass | 410.24302422 g/mol |
| Topological Polar Surface Area | 96.6 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 558 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
