
1. Dyrenium
2. Dytac
3. Urocaudal
1. 396-01-0
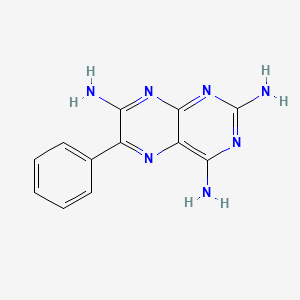
2. 6-phenylpteridine-2,4,7-triamine
3. 2,4,7-triamino-6-phenylpteridine
4. Dyrenium
5. Dytac
6. Pterofen
7. Pterophene
8. Triamteren
9. Triamteril
10. Triteren
11. Ademin
12. Ademine
13. Diurene
14. Noridil
15. Taturil
16. Teridin
17. Urocaudal
18. Jatropur
19. Noridyl
20. Triampur
21. Diren
22. Ditak
23. Dyren
24. Teriam
25. Tri-span
26. Triamteril Complex
27. Trispan
28. 6-phenyl-2,4,7-pteridinetriamine
29. 2,4,7-pteridinetriamine, 6-phenyl-
30. Sk&f 8542
31. 6-phenyl-2,4,7-triaminopteridine
32. Skf 8542
33. Pteridine, 2,4,7-triamino-6-phenyl-
34. 2,4,7-triamino-6-fenilpteridina
35. Nsc 77625
36. Nsc-77625
37. Sk-8542
38. Brn 0266723
39. Diucelpin
40. Sk&f-8542
41. Ws821z52lq
42. Masuharmin
43. Triamizide
44. Triamthiazid
45. Amteren
46. Dinazide
47. Diutensat
48. Diuteren
49. Dyberzide
50. Dytenzide
51. Esiteren
52. Hidiurese
53. Hydrene
54. Hypertorr
55. Jenateren
56. Kalspare
57. Nephral
58. Renezide
59. Reviten
60. Tricilone
61. Triurene
62. Uretren
63. Diarol
64. Isobar
65. Trizid
66. Anjal
67. Dazid
68. Turfa
69. Apo-triazide
70. Thiazid Wolff
71. Nci-c56042
72. Nsc77625
73. Ademin(e)
74. Nsc-639359
75. Ncgc00016016-10
76. Triamterena
77. Triamterenum
78. Cas-396-01-0
79. Triazide
80. Fluss 40
81. Sali-puren
82. Dsstox_cid_1373
83. Dsstox_rid_76117
84. Dsstox_gsid_21373
85. Triamterenum [inn-latin]
86. Triamterena [inn-spanish]
87. Dyrenium (tn)
88. Ccris 5872
89. Pteridine Deriv. 11
90. Hsdb 3405
91. 2,4,7-triamino-6-fenilpteridina [italian]
92. Nci C56042
93. Sr-01000002968
94. Einecs 206-904-3
95. Nsc 639359
96. Unii-ws821z52lq
97. Ai3-60017
98. Prestwick_480
99. Mfcd00006708
100. Dyazide (salt/mix)
101. Triamterene [usan:usp:inn:ban:jan]
102. Spectrum_000508
103. Triamterene, >=99%
104. Triamterene [mi]
105. Prestwick0_000034
106. Prestwick1_000034
107. Prestwick2_000034
108. Prestwick3_000034
109. Spectrum2_000938
110. Spectrum3_001372
111. Spectrum4_000366
112. Spectrum5_001034
113. Lopac-t-4143
114. Triamterene [inn]
115. Triamterene [jan]
116. Triamterene [hsdb]
117. Triamterene [iarc]
118. Triamterene [usan]
119. Chembl585
120. T 4143
121. Triamterene [vandf]
122. Nciopen2_004741
123. Triamterene [mart.]
124. Lopac0_001196
125. Oprea1_825704
126. Schembl40707
127. Bspbio_000127
128. Bspbio_002924
129. Kbiogr_000831
130. Kbioss_000988
131. Triamterene [usp-rs]
132. Triamterene [who-dd]
133. 5-26-17-00447 (beilstein Handbook Reference)
134. Mls000069431
135. Bidd:gt0534
136. Divk1c_000433
137. Spectrum1500589
138. Spbio_000876
139. Spbio_002048
140. Bdbm6644
141. Bpbio1_000141
142. Chebi:9671
143. Gtpl4329
144. Triamterene (jp17/usp/inn)
145. 2,7-triamino-6-phenylpteridine
146. 6-phenyl-2,7-triaminopteridine
147. Dtxsid6021373
148. Hms501f15
149. Kbio1_000433
150. Kbio2_000988
151. Kbio2_003556
152. Kbio2_006124
153. Kbio3_002144
154. Skf8542
155. Triamterene [orange Book]
156. Ninds_000433
157. Hms1568g09
158. Hms2092o17
159. Hms2095g09
160. Hms2232b04
161. Hms3259c08
162. Hms3263p13
163. Hms3371d10
164. Hms3652e10
165. Hms3712g09
166. Pharmakon1600-01500589
167. Triamterene [ep Monograph]
168. Triamterene [usp Impurity]
169. Zinc120286
170. 2,7-pteridinetriamine, 6-phenyl-
171. Pteridine,4,7-triamino-6-phenyl-
172. Triamterene [usp Monograph]
173. Bcp28855
174. Dyazide Component Triamterene
175. Hy-b0575
176. Maxzide Component Triamterene
177. Tox21_110283
178. Tox21_202021
179. Tox21_302833
180. Tox21_501196
181. Ccg-40090
182. Nsc639359
183. Nsc757367
184. S4080
185. Stk300348
186. Akos003790819
187. Tox21_110283_1
188. Db00384
189. Lp01196
190. Nc00544
191. Nsc-757367
192. Sdccgsbi-0051163.p004
193. Triamterene Component Of Dyazide
194. Triamterene Component Of Maxzide
195. Idi1_000433
196. Smp1_000147
197. Ncgc00016016-01
198. Ncgc00016016-02
199. Ncgc00016016-03
200. Ncgc00016016-04
201. Ncgc00016016-05
202. Ncgc00016016-06
203. Ncgc00016016-07
204. Ncgc00016016-08
205. Ncgc00016016-09
206. Ncgc00016016-11
207. Ncgc00016016-12
208. Ncgc00016016-13
209. Ncgc00016016-14
210. Ncgc00016016-15
211. Ncgc00016016-16
212. Ncgc00016016-18
213. Ncgc00016016-28
214. Ncgc00016016-29
215. Ncgc00023458-03
216. Ncgc00023458-04
217. Ncgc00023458-05
218. Ncgc00023458-06
219. Ncgc00023458-07
220. Ncgc00256495-01
221. Ncgc00259570-01
222. Ncgc00261881-01
223. Ac-14066
224. As-12471
225. Smr000059118
226. Sbi-0051163.p003
227. Db-049442
228. Ab00052116
229. B2275
230. Bb 0256885
231. Eu-0101196
232. Sw196688-3
233. T1288
234. Triamterene 1.0 Mg/ml In Dimethyl Sulfoxide
235. D00386
236. D95706
237. Wln: T66 Bn Dn Gn Jnj Cz Ez Hr& Iz
238. Ab00052116_13
239. Ab00052116_14
240. 396t010
241. A824641
242. Q221520
243. Sr-01000002968-2
244. Sr-01000002968-4
245. Sr-01000002968-6
246. Brd-k92049597-001-05-9
247. Brd-k92049597-001-10-9
248. Z275128596
249. Triamterene, British Pharmacopoeia (bp) Reference Standard
250. Triamterene, European Pharmacopoeia (ep) Reference Standard
251. Triamterene, United States Pharmacopeia (usp) Reference Standard
252. Triamterene, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 253.26 g/mol |
|---|---|
| Molecular Formula | C12H11N7 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 253.10759338 g/mol |
| Monoisotopic Mass | 253.10759338 g/mol |
| Topological Polar Surface Area | 130 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 307 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Dyrenium |
| PubMed Health | Triamterene (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Each capsule for oral use, with opaque red cap and body, contains Triamterene USP, 50 or 100 mg, and is imprinted with the product name, DYRENIUM, strength (50 mg or 100 mg) and WPC 002 (for the 50-mg strength) and WPC 003 (for the 100-mg strength).... |
| Active Ingredient | Triamterene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Wellspring Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Dyrenium |
| PubMed Health | Triamterene (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Each capsule for oral use, with opaque red cap and body, contains Triamterene USP, 50 or 100 mg, and is imprinted with the product name, DYRENIUM, strength (50 mg or 100 mg) and WPC 002 (for the 50-mg strength) and WPC 003 (for the 100-mg strength).... |
| Active Ingredient | Triamterene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Wellspring Pharm |
Diuretics; Epithelial Sodium Channel Blockers
National Library of Medicine's Medical Subject Headings. Triamterene. Online file (MeSH, 2018). Available from, as of August 29, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Triamterene is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 29, 2018: https://clinicaltrials.gov/
Dyrenium (triamterene) is indicated in the treatment of edema associated with congestive heart failure, cirrhosis of the liver and the nephrotic syndrome; steroid-induced edema, idiopathic edema and edema due to secondary hyperaldosteronism. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Dyrenium (Triamterene Capsule) (Updated: August 7, 2017). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebb177c0-d45d-4443-a85a-e76bd9931a42
Dyrenium may be used alone or with other diuretics, either for its added diuretic effect or its potassium-sparing potential. It also promotes increased diuresis when patients prove resistant or only partially responsive to thiazides or other diuretics because of secondary hyperaldosteronism. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Dyrenium (Triamterene Capsule) (Updated: August 7, 2017). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebb177c0-d45d-4443-a85a-e76bd9931a42
For more Therapeutic Uses (Complete) data for Triamterene (6 total), please visit the HSDB record page.
/BOXED WARNING/ Warnings: Abnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-sparing agents, including Dyrenium. Hyperkalemia is more likely to occur in patients with renal impairment and diabetes (even without evidence of renal impairment), and in the elderly or severely ill. Since uncorrected hyperkalemia may be fatal, serum potassium levels must be monitored at frequent intervals especially in patients receiving Dyrenium, when dosages are changed or with any illness that may influence renal function.
NIH; DailyMed. Current Medication Information for Dyrenium (Triamterene Capsule) (Updated: August 7, 2017). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebb177c0-d45d-4443-a85a-e76bd9931a42
Dyrenium should not be given to patients receiving other potassium-sparing agents, such as spironolactone, amiloride hydrochloride, or other formulations containing triamterene. Two deaths have been reported in patients receiving concomitant spironolactone and Dyrenium or Dyazide. Although dosage recommendations were exceeded in one case and in the other serum electrolytes were not properly monitored, these two drugs should not be given concomitantly.
NIH; DailyMed. Current Medication Information for Dyrenium (Triamterene Capsule) (Updated: August 7, 2017). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebb177c0-d45d-4443-a85a-e76bd9931a42
Dyrenium (triamterene) should not be used in patients with pre-existing elevated serum potassium, as is sometimes seen in patients with impaired renal function or azotemia, or in patients who develop hyperkalemia while on the drug. Patients should not be placed on dietary potassium supplements, potassium salts or potassium-containing salt substitutes in conjunction with Dyrenium.
NIH; DailyMed. Current Medication Information for Dyrenium (Triamterene Capsule) (Updated: August 7, 2017). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebb177c0-d45d-4443-a85a-e76bd9931a42
Potassium loss has been reported during triamterene therapy in some patients with hepatic cirrhosis and may result in signs and symptoms of hepatic coma or precoma. Serum potassium concentrations should be closely monitored in patients with hepatic cirrhosis and potassium supplementation administered if required.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2877
For more Drug Warnings (Complete) data for Triamterene (25 total), please visit the HSDB record page.
Triamterene is indicated for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and the nephrotic syndrome; also in steroid-induced edema, idiopathic edema, and edema due to secondary hyperaldosteronism. Triamterene in combination with hydrochlorothiazide is indicated for the managment of hypertension or treatment of edema in patients who develop hypokalemia following hydrochlorothiazide monotherapy, and in patients who require thiazide diuretic and in whom the development of hypokalemia cannot be risked. Triamterene allows the maintenance of potassium balance when given in combination with loop diuretics and thiazides.
Triamterene, a relatively weak, potassium-sparing diuretic and antihypertensive, is used in the management of hypertension and edema. It primarily works on the distal nephron in the kidneys; it acts from the late distal tubule to the collecting duct to inhibit Na+ reabsorption and decreasing K+ excretion. As triamterene tends to conserve potassium more strongly than promoting Na+ excretion, it can cause an increase in serum potassium, which may result in hyperkalemia potentially associated with cardiac irregularities. In healthy volunteers administered with oral triamterene, there was an increase in the renal clearnace of sodium and magnesium, and a decrease in the clearance of uric acid and creatinine due to its effect of reducing glomerular filtration renal plasma flow. Triamterene does not affect calcium excretion. In clinical trials, the use of triamterene in combination with hydrochlorothiazide resulted an enhanced blood pressure-lowering effects of hydrochlorothiazide.
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
Epithelial Sodium Channel Blockers
A subclass of sodium channel blockers that are specific for EPITHELIAL SODIUM CHANNELS. (See all compounds classified as Epithelial Sodium Channel Blockers.)
C - Cardiovascular system
C03 - Diuretics
C03D - Aldosterone antagonists and other potassium-sparing agents
C03DB - Other potassium-sparing agents
C03DB02 - Triamterene
Absorption
Triamterene is shown to be rapidly absorbed in the gastrointestinal tract Its onset of action achiveved within 2 to 4 hours after oral ingestion and its duration of action is 12-16 hours. It is reported that the diuretic effect of triamterene may not be observed for several days after administration. In a pharmacokinetic study, the oral bioavailability of triamterene was determined to be 52%. Following administration of a single oral dose to fasted healthy male volunteers, the mean AUC of triamterene was about 148.7 ng*hr/mL and the mean peak plasma concentrations (Cmax) were 46.4 ng/mL reached at 1.1 hour after administration. In a limited study, administration of triamterene in combination with hydrochlorothiazide resulted in an increased bioavailability of triamterene by about 67% and a delay of up to 2 hours in the absorption of the drug. It is advised that triamterene is administered after meals; in a limited study, combination use of triamterene and hydrochlorothiazide with the consumption of a high-fat meal resulted in an increase in the mean bioavailability and peak serum concentrations of triamterene and its active sulfate metabolite, as well as a delay of up to 2 hours in the absorption of the active constituents.
Route of Elimination
Triamterene and its metabolites are excreted by the kidney by filtration and tubular secretion. Upon oral ingestion, somewhat less than 50% of the oral dose reaches the urine. About 20% of an oral dose appears unchanged in the urine, 70% as the sulphate ester of hydroxytriamterene and 10% as free hydroxytriamterene and triamterene glucuronide.
Volume of Distribution
In a pharmacolinetic study involving healthy volunteers receiving triamterene intravenously, the volumes of distribution of the central compartment of triamterene and its hydroxylated ester metabolite were 1.49 L/kg and 0.11 L/kg, respectively. Triamterene was found to cross the placental barrier and appear in the cord blood of animals.
Clearance
The total plasma clearance was 4.5 l/min and renal plasma clearance was 0.22 l/kg following intravenous administration of triamterene in healthy volunteers.
Earlier in vivo studies revealed a low concentration of triamterene in the brain of guinea pigs and baboons, and a transfer of the drug from the fetus to the mother. Additional investigations have been performed to characterize further the transport system(s) for triamterene in the central nervous system (CNS), placenta, and kidney. In guinea pigs a very low brain to free plasma concentration ratio (0.1) was achieved 3.5 min after drug administration and was maintained during 180 min of drug infusion. The cerebrospinal fluid (CSF) concentration was similar to the concentration of the drug in the brain. A higher brain to free plasma concentration ratio was gradually reached in dogs studied with nanogram per ml and microgram per ml concentrations of triamterene in CSF. Administration of triamterene to fetal and maternal sheep revealed a placental extraction (E) from fetal plasma to placenta 20 times greater than that from maternal plasma to placenta. The E from fetal plasma to placenta was unaffected by a triamterene concentration in the maternal circulation 10 times that in the fetus. These findings and studies of renal clearance support an active transfer of triamterene by the CNS, placenta, and kidney; the physiologic substrate for these systems is unknown.
PMID:234832 Pruitt AW et al; Drug Metab Dispos 3 (1): 30-41 (1975)
The kinetics of triamterene and its active phase II metabolite were studied in 32 patients with various degrees of impaired renal function; the creatinine clearances ranged from 135 to 10 mL/min. The area under the plasma concentration-time curves (AUC) for triamterene were not influenced by kidney function, but the AUCs for the effective metabolite OH-TA-ester were significantly elevated in renal failure, indicating accumulation of the metabolite. Urinary recovery of triamterene and its metabolite over a 48 hr collection period was significantly reduced in renal failure. This is considered to be due to delayed urinary excretion, corresponding to reduced renal clearance. The renal clearance of the native drug exceeded that of the metabolite, because of their different protein binding, 55% for triamterene and 91% for the metabolite. The latter is eliminated almost exclusively via tubular secretion and extra-renal elimination is less important. ...
PMID:6861860 Knauf H et al; Eur J Clin Pharmacol 24 (4): 453-6 (1983)
Although renal elimination is only a minor route of excretion for triamterene, it is the main route of elimination of 4'-hydroxytriamterene sulfate. Thus, in individuals with renal impairment, accumulation of the sulfate is substantial and progressive, but negligible for triamterene. The kinetics of triamterene were observed in 32 patients with widely varying degrees of creatinine clearance (10-135 mL/minute), an indicator of renal function. In patients with reduced renal function, significant accumulation in plasma and reduced renal clearance of the sulfate were reported. Plasma concentrations of the parent drug were not increased.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 278 (2016)
Patients with liver cirrhosis have reduced ability to hydroxylate triamterene, as evidenced by high plasma concentrations of triamterene and low concentrations of 4'-hydroxytriamterene sulfate. After administration of 200 mg of triamterene, peak plasma concentrations in eight patients without liver disease were 559 +/- 48 ng/mL and 2956 +/- 320 ng/mL for triamterene and 4'-hydroxytriamterene sulfate, respectively. In the seven patients with alcoholic cirrhosis, peak plasma concentrations of triamterene were increased to 1434 +/- 184 ng/mL, while the concentrations of the sulfate were reduced to 469 +/- 84 ng/mL. Renal clearance was also reduced in patients with cirrhosis: the clearance of triamterene and the sulfate were 2.8 +/- 0.7 and 38.0 +/- 6.6 mL/minute, respectively, compared with 14.4 +/- 1.5 and 116.7 +/- 11.6 mL/ minute, respectively, in patients without liver disease.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 278 (2016)
For more Absorption, Distribution and Excretion (Complete) data for Triamterene (12 total), please visit the HSDB record page.
Triamterene undergoes phase I metabolism involving hydroxylation, via CYP1A2 activity, to form 4'-hydroxytriamterene. 4'-Hydroxytriamterene is further transformed in phase II metabolism mediated by cytosolic sulfotransferases to form the major metabolite, 4-hydroxytriamterene sulfate, which retains a diuretic activity. Both the plasma and urine levels of this metabolite greatly exceed triamterene levels while the renal clearance of the sulfate conjugate was les than that of triamterene; this low renal clearance of the sulfate conjugate as compared with triamterene may be explained by the low unbound fraction of the metabolite in plasma.
The metabolic and excretory fate of triamterene has not been fully determined. The drug is reportedly metabolized to 6-p-hydroxytriamterene and its sulfate conjugate.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2878
The kinetics of triamterene and its active phase II metabolite were studied in 32 patients with various degrees of impaired renal function; the creatinine clearances ranged from 135 to 10 mL/min. The area under the plasma concentration-time curves (AUC) for triamterene were not influenced by kidney function, but the AUCs for the effective metabolite OH-TA-ester were significantly elevated in renal failure, indicating accumulation of the metabolite. Urinary recovery of triamterene and its metabolite over a 48 hr collection period was significantly reduced in renal failure. This is considered to be due to delayed urinary excretion, corresponding to reduced renal clearance. The renal clearance of the native drug exceeded that of the metabolite, because of their different protein binding, 55% for triamterene and 91% for the metabolite. The latter is eliminated almost exclusively via tubular secretion and extra-renal elimination is less important. ...
PMID:6861860 Knauf H et al; Eur J Clin Pharmacol 24 (4): 453-6 (1983)
Patients with liver cirrhosis have reduced ability to hydroxylate triamterene, as evidenced by high plasma concentrations of triamterene and low concentrations of 4'-hydroxytriamterene sulfate. After administration of 200 mg of triamterene, peak plasma concentrations in eight patients without liver disease were 559 +/- 48 ng/mL and 2956 +/- 320 ng/mL for triamterene and 4'-hydroxytriamterene sulfate, respectively. In the seven patients with alcoholic cirrhosis, peak plasma concentrations of triamterene were increased to 1434 +/- 184 ng/mL, while the concentrations of the sulfate were reduced to 469 +/- 84 ng/mL. Renal clearance was also reduced in patients with cirrhosis: the clearance of triamterene and the sulfate were 2.8 +/- 0.7 and 38.0 +/- 6.6 mL/minute, respectively, compared with 14.4 +/- 1.5 and 116.7 +/- 11.6 mL/ minute, respectively, in patients without liver disease.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 278 (2016)
The half-life of the drug in plasma ranges from 1.5 to 2 hours. In a pharmacokinetic study involving healthy volunteers, the terminal half-lives for triamterene and 4-hydroxytriamterene sulfate were 255 42 and 188 70 minutes, respectively, after intravenous infusion of the parent drug.
The plasma half-life of triamterene is 100-150 minutes.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2878
Triamterene inhibits the epithelial sodium channels (ENaC) located on the lumenal side in the late distal convoluted tubule and collecting tubule, which are transmembrane channels that normally promotes sodium uptake and potassium secretion. In the late distal tubule to the collecting duct, sodium ions are actively reabsorbed via ENaC on the lumnial membrane and are extruded out of the cell into the peritubular medium by a sodium-potassium exchange pump, the Na-K-ATPase, with water following passively. Triamterene exerts a diuretic effect on the distal renal tubule to inhibit the reabsorption of sodium ions in exchange for potassium and hydrogen ions and its natriuretic activity is limited by the amount of sodium reaching its site of action. Its action is antagonistic to that of adrenal mineralocorticoids, such as aldosterone, but it is not an inhibitor or antagonist of aldosterone. Triamterene maintains or increases the sodium excretionm, thereby increasing the excretion of water, and reduces the excess loss of potassium, hydrogen and chloride ions by inhibiting the distal tubular exchange mechanism. Due to its diuretic effect, triamterene rapidly and reversibly reduces the lumen-negative transepithelial potential difference by almost complete abolition of Na+ conductance without altering K+ conductance. This reduces the driving force for potassium movement into the tubular lumen and thus decreases potassium excretion. Triamterene is similar in action to [amiloride] but, unlike amiloride, increases the urinary excretion of magnesium.