


1. 232925-18-7
2. 2zrz4tsw3f
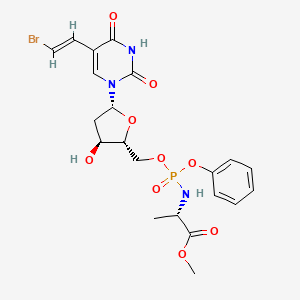
3. Methyl (2s)-2-[[[(2r,3s,5r)-5-[5-[(e)-2-bromoethenyl]-2,4-dioxopyrimidin-1-yl]-3-hydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate
4. Nb-1011
5. L-alanine, N-(5-((1e)-2-bromoethenyl)-2'-deoxy-p-phenyl-5'-uridylyl)-, Methyl Ester
6. Bvdu Prodrug
7. (2s)-methyl 2-(((((2r,3s,5r)-5-(5-((e)-2-bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)-3-hydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
8. Brivudine Phosphoramidate
9. Unii-2zrz4tsw3f
10. Schembl13904642
11. Dtxsid00177862
12. 5-(2-bromovinyl)-2'-deoxy-5'-uridyl-phenyl-alanylphosphoramidate, Trans-
13. Db05116
14. (e)-5-(2-bromovinyl)-2'-deoxy-5'-uridyl Phenyl L-methoxyalaninylphosphoramidate
15. E-5-(2-bromovinyl)-2'-deoxyuridine-5'-(l-methylalaninyl)-phenylphosphoramidate
16. Hy-106168
17. Cs-0025066
18. J3.616.614b
19. Q7799611
20. (s)-2-({(2r,3s,5r)-5-[5-((e)-2-bromo-vinyl)-2,4-dioxo-3,4-dihydro-2h-pyrimidin-1-yl]-3-hydroxy-tetrahydro-furan-2-ylmethoxy}-phenoxy-phosphorylamino)-propionic Acid Methyl Ester
21. Methyl (2s)-2-[[[(2r,3s,5r)-5-[5-[(e)-2-bromovinyl]-2,4-dioxo-pyrimidin-1-yl]-3-hydroxy-tetrahydrofuran-2-yl]methoxy-phenoxy-phosphoryl]amino]propanoate
| Molecular Weight | 574.3 g/mol |
|---|---|
| Molecular Formula | C21H25BrN3O9P |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 573.05118 g/mol |
| Monoisotopic Mass | 573.05118 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 909 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in colorectal cancer.
NB1011 targets thymidylate synthase (TS), which catalyzes the transformation of E-5-(2-bromovinyl)-2'-deoxyuridine-5'-monophosphate (BVdUMP) into cytotoxic reaction products. Due to the elevated levels of TS expression in tumor cells compared to normal cells, these cytotoxic products are preferentially generated inside tumor cells, and, as expected, NB1011 is more toxic to cells with higher levels of TS expression. Therefore, NB1011 therapy should kill tumor cells without severely damaging normal cells.
