
1. Meballymal
2. Quinalbarbitone
3. Sebar
4. Secobarbital Sodium
5. Seconal
6. Seconal Sodium
7. Sodium, Secobarbital
1. Quinalbarbitone
2. Secobarbitone
3. Seconal
4. Meballymal
5. Quinalbarbital
6. Hypotrol
7. Evronal
8. 76-73-3
9. Immenox
10. Secobarbitalum
11. Barbosec
12. (+-)-secobarbital
13. Secobarbitale [dcit]
14. Somatarax
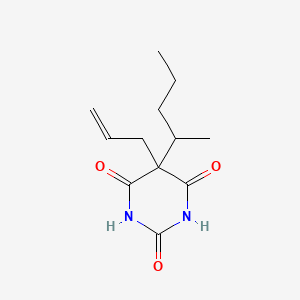
15. 5-allyl-5-(1-methylbutyl)barbituric Acid
16. Secobarbitalum [inn-latin]
17. (rs)-secobarbital
18. 5-allyl-5-(1-methylbutyl)malonylurea
19. Barbituric Acid, 5-allyl-5-(1-methylbutyl)-
20. Secobarbital Cii
21. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylbutyl)-5-(2-propenyl)-
22. 5-pentan-2-yl-5-prop-2-enyl-1,3-diazinane-2,4,6-trione
23. (+/-)-secobarbital
24. (+-)-5-allyl-5-(1-methylbutyl)-barbituric Acid
25. Chebi:9073
26. Meballymalum
27. Somosal
28. 5-allyl-5-(1-methylbutyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
29. 1p7h87in75
30. Dea No. 2315
31. Secobarbitale
32. Hyptran
33. 5-allyl-5-(1-methylbutyl)pyrimidine-2,4,6(1h,3h,5h)-trione
34. Barbituric Acid, 5-allyl-5-(1-methylbutyl)-, (+-)-
35. Sodium Secobarbital
36. 5-(1-methylbutyl)-5-(2-propenyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
37. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylbutyl)-5-(2-propen-1-yl)-
38. Sodium Quinalbarbitone
39. 5-allyl-5-(1-methylbutyl)barbiturate
40. Seconal (tn)
41. Hsdb 3182
42. (+/-)-quinalbarbitone
43. Secobarbital Suppository Dosage Form
44. Einecs 200-982-2
45. Secobarbital (usp/inn)
46. Secobarbital [usp:inn]
47. Brn 0225330
48. Unii-1p7h87in75
49. Ncgc00247712-01
50. Hyptran (salt/mix)
51. Imesonal (salt/mix)
52. Trisomnin (salt/mix)
53. Immenoctal (salt/mix)
54. Secobarbital [inn]
55. Chembl447
56. Secobarbital [hsdb]
57. (.+/-.))-secobarbital
58. Secobarbital [vandf]
59. Schembl80734
60. Secobarbital [mart.]
61. Secobarbital Sodium Free Acid
62. 29071-21-4
63. 5-24-09-00235 (beilstein Handbook Reference)
64. Secobarbital [usp-rs]
65. Secobarbital [who-dd]
66. Gtpl7615
67. Dtxsid6044145
68. Schembl11110602
69. Secobarbital, Analytical Standard
70. Secobarbital [usp Impurity]
71. Secobarbital 0.1 Mg/ml In Methanol
72. Secobarbital 1.0 Mg/ml In Methanol
73. 5-(pentan-2-yl)-5-(prop-2-en-1-yl)-1,3-diazinane-2,4,6-trione
74. 5-(1-methylbutyl)-5-(2-propen-1-yl)-2,4,6(1h,3h,5h)-pyrimidinetrione
75. 5-(pentan-2-yl)-5-(prop-2-en-1-yl)-pyrimidine-2,4,6(1h,3h,5h)-trione
76. Db00418
77. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylbutyl)-5-(2-propenyl)-, (+-)-
78. Secobarbital Sodium Free Acid [mi]
79. 5-allyl-5-(1-methylbutyl)-barbituric Acid
80. S0223
81. D00430
82. Q414788
83. Secobarbital Suppository Dosage Form [dea No. 2316]
84. Secobarbital, United States Pharmacopeia (usp) Reference Standard
85. 2,4,6(1h,3h,5h)-pyrimidinitrione, 5-(1-methylbutyl)-5-(2-propenyl)-
86. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylbutyl)-5-(2-propenyl)-, (+-)- (9ci)
87. Secobarbital Solution, 1 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
88. Secobarbital Solution, 1.0 Mg/ml In Methanol, Analytical Standard, For Drug Analysis
| Molecular Weight | 238.28 g/mol |
|---|---|
| Molecular Formula | C12H18N2O3 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 238.13174244 g/mol |
| Monoisotopic Mass | 238.13174244 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjuvants, Anesthesia; GABA Modulators; Sedatives, Barbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Hypnotic, for the short-term treatment of insomnia, since it appears to lose its effectiveness for sleep induction and sleep maintenance after 2 weeks. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
Preanesthetic. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
Medication (Vet): sodium salt is used. Use: as rapid acting sedative or hypnotic; occasionally as preanesthetic; and as anesthetic. /Secobarbital sodium/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 521
The hypnotic effectiveness of this barbiturate is comparable to pentobarbital sodium; secobarbital is not an antianxiety agent. It is usually given orally. Parenteral routes should be used to induce sleep only when oral admin is impossible or impractical. ... Used more frequently for hypnosis than for sedation but may lose its effectiveness by 2nd wk of continual admin. /Secobarbital sodium/
American Medical Association, Department of Drugs. Drug Evaluations. 6th ed. Chicago, Ill: American Medical Association, 1986., p. 105
The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated.
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs.
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
Complex behaviors such as "sleep-driving" (ie, driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naive as well as in sedative-hypnotic-experienced persons. ... Other complex behaviors (eg, preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007).
For injection, an aq soln is preferred to preparations containing polyethylene glycol because the latter may be irritating to the kidney, especially in patients with renal insufficiency. /Secobarbital sodium/
American Medical Association, Department of Drugs. Drug Evaluations. 6th ed. Chicago, Ill: American Medical Association, 1986., p. 105
For more Drug Warnings (Complete) data for Secobarbital (33 total), please visit the HSDB record page.
In general, an oral dose of 1 g of most barbiturates produces serious poisoning in an adult. Death commonly occurs after 2 to 10 g of ingested barbiturate. The sedated, therapeutic blood levels of secobarbital range between 0.5 to 5 ug/mL; the usual lethal blood level ranges from 15 to 40 ug/mL.
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
The toxic dose of barbiturates varies considerably but, in general, a severe reaction is likely to occur when the amount ingested is more than 10 times the usual oral hypnotic dose. Potentially lethal blood concentrations are those in excess of 80 ug/mL for phenobarbital, 50 ug/mL for amobarbital or butabarbital, and approximately 30 ug/mL for secobarbital or pentobarbital; however, some patients have survived much higher blood concentrations. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2578
For the Short-term treatment of intractable insomnia for patients habituated to barbiturates
Secobarbital, a barbiturate, is used for the induction of anesthesia prior to the use of other general anesthetic agents and for induction of anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA06 - Secobarbital
Route of Elimination
Barbiturates are metabolized primarily by the hepatic microsomal enzyme system, and the metabolic products are excreted in the urine and, less commonly, in the feces.
Approximately 90% of an oral dose of secobarbital is absorbed from the GI tract within 2 hours after ingestion, and peak plasma concentrations are reached within 2-4 hours. Virtually all of the drug is absorbed following rectal administration.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
Plasma secobarbital concentrations of 1-5 ug/mL generally produce sedation, and plasma concentrations of 5-15 ug/mL produce sleep in most patients; however, plasma concentrations of greater than 10 ug/mL may produce coma. Plasma concentrations in excess of 30 ug/mL are potentially lethal.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
When hypnotic doses of secobarbital are administered orally, the onset of action usually occurs within 15 minutes. Full hypnotic effect of the drug usually occurs 15-30 minutes following oral or rectal administration, 7-10 minutes following im administration, and 1-3 minutes following iv administration. (Rectal and parenteral preparations of the drug are no longer commercially available in the US.) The duration of the hypnotic effect is 1-4 hours following oral or rectal administration of an average dose of secobarbital and about 15 minutes following iv administration of a 100- to 150-mg dose.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
Approximately 30-45% of the drug is bound to plasma proteins.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
For more Absorption, Distribution and Excretion (Complete) data for Secobarbital (18 total), please visit the HSDB record page.
Secobarbital is metabolized by the liver via penultimate oxidation of the 1-methylbutyl substituent to form 5-allyl-5(3'-hydroxy-1'-methylbutyl)barbituric acid (hydroxysecobarbital), the major metabolite. Oxidation of the allyl substituent may also occur to form 5-[2',3'-dihydroxypropyl-5-(1'-methylbutyl)]barbituric acid (secodiol). These inactive metabolites are excreted in urine unchanged or as glucuronide conjugates. Hydroxysecobarbital may also be further oxidized to a ketone or a carboxylic acid. The allyl substituent at C 5 may be removed, and small quantities of the resulting 5-(1'-methylbutyl)barbituric acid metabolite have been found in urine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
Differences were observed in urine profiles from the rat and guinea pig following ip admin of secobarbital. ... The rat hydroxylated secobarbital on the side-chain and produced only a small amount of the diol, a product of epoxidation and hydrolysis of the allyl side-chain. These metabolites were excreted in almost equal quantities by the guinea pig. The ratio of metabolites was independent of the dose in the range 20-100 mg/kg-l in the rat. Doses over 50 mg/kg-l were lethal in the guinea pig.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 163
Both the allyl and sec-amyl side-chains undergo metabolism to give mainly seconal diol and 2 diastereoisomers of hydroxyseconal, in man and dog; and hydroxyseconal and seconal carboxylic acid in rabbit.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 186
In dogs and in man, secobarbital ... is metabolized into 5-(2,3-dihydroxypropyl)-5-(1-methylbutyl)barbituric acid and (+-)5-allyl-5-(3-hydroxy-1-methylbutyl)barbituric acid ... 5-(1-methylbutyl)barbituric acid was found in urine of some human subjects and ... in extracts from the liver of a suicide.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 152
For more Metabolism/Metabolites (Complete) data for Secobarbital (7 total), please visit the HSDB record page.
The plasma half-life for secobarbital sodium in adults ranges between 15 to 40 hours, with a mean of 28 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Seconal sodium (secobarbital sodium) capsule (May 2007). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5171
Secobarbital has an elimination half-life of about 30 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2585
Secobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.
The exact mechanism(s) by which barbiturates exert their effect on the CNS, has not been fully elucidated. However, it is believed that such effects are related, at least partially, to the drugs' ability to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS, by altering inhibitory synaptic transmissions that are mediated by GABA receptors. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticular formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Relatively low doses of the barbiturates depress the sensory cortex, decrease motor activity, and produce sedation and drowsiness. In some patients, however, drowsiness may be preceded by a period of transient elation, confusion, euphoria, or excitement, especially after subhypnotic doses of aprobarbital, pentobarbital, or secobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Larger doses distort judgment, cloud perception, suppress motor activity, and produce drowsiness and sleep. Still larger doses induce anesthesia. Barbiturate-induced sleep differs from physiologic sleep. Barbiturates reduce the rapid eye movement (REM) or dreaming stage of sleep. Stages III and IV sleep are also decreased. Although tolerance develops to the REM-suppressant effects during chronic administration, REM rebound occurs when the drugs are withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Mechanism of Action (Complete) data for Secobarbital (15 total), please visit the HSDB record page.