

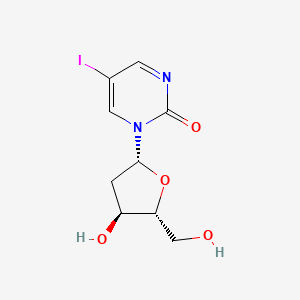

1. 1-(2-deoxy-beta-ribofuranosyl)-5-iodo-2-pyrimidinone
2. 5-iodo-2-pyrimidinone 2' Deoxyribonucleoside
3. 5-iodo-2-pyrimidinone-2'-deoxyribose
4. Ipdr
1. 93265-81-7
2. Ipdr
3. 5-iodo-2-pyrimidinone-2'-deoxyribose
4. Ipd-r
5. 3hx21a3sqf
6. 1-(2-deoxy-beta-ribofuranosyl)-5-iodo-2-pyrimidinone
7. Nsc-726188
8. 2(1h)-pyrimidinone, 1-(2-deoxy-beta-d-erythro-pentofuranosyl)-5-iodo-
9. 1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-iodopyrimidin-2(1h)-one
10. Ropidoxuridine [usan]
11. Unii-3hx21a3sqf
12. Ropidoxuridine (inn/usan)
13. Ropidoxuridine [usan:inn]
14. 5-iodo-2-pyrimidinone 2' Deoxyribonucleoside
15. Ropidoxuridine [inn]
16. Schembl8602575
17. Chembl2103821
18. Dtxsid00239353
19. Zinc6091380
20. 5-iodo-2-pyrimidinone-2-deoxyribose
21. Db06485
22. 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidin-2-one
23. Hy-13742
24. Cs-0007768
25. D08992
26. D96122
27. 265i817
28. A901121
29. Q27257237
30. 5-iodo-1-(2-deoxy-beta-d-ribofuranosyl)pyrimidin-2(1h)-one
31. 1-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-5-iodopyrimidin-2(1h)-one
32. 2(1h)-pyrimidinone, 1-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-5-iodo-
| Molecular Weight | 338.10 g/mol |
|---|---|
| Molecular Formula | C9H11IN2O4 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 337.97635 g/mol |
| Monoisotopic Mass | 337.97635 g/mol |
| Topological Polar Surface Area | 82.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 357 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in cancer/tumors (unspecified).
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
