


1. Kisqali
2. Lee011
3. Ribociclib
1. Lee011 Succinate
2. 1374639-75-4
3. Lee-011 Succinate
4. Lee011-bba
5. Ribociclib Succinate [usan]
6. Kisqali
7. Lee-011-bba
8. Bg7hlx2919
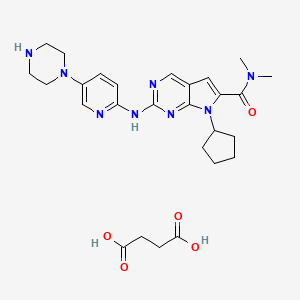
9. 7-cyclopentyl-n,n-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7h-pyrrolo[2,3-d]pyrimidine-6-carboxamide Succinate
10. Butanedioic Acid;7-cyclopentyl-n,n-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo[2,3-d]pyrimidine-6-carboxamide
11. Ribociclib Succinate (usan)
12. Butanedioic Acid, Compd. With 7-cyclopentyl-n,n-dimethyl-2-((5-(1-piperazinyl)-2-pyridinyl)amino)-7h-pyrrolo(2,3-d)pyrimidine-6-carboxamide (1:1)
13. Butanedioic Acid, Compd. With 7-cyclopentyl-n,n-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-7h-pyrrolo[2,3-d]pyrimidine-6-carboxamide (1:1)
14. Unii-bg7hlx2919
15. Kisqali (tn)
16. Lee011 (succinate)
17. Birociclib [who-dd]
18. Schembl2684999
19. Chembl3707266
20. Ribociclib Succinate [mi]
21. Dtxsid301027923
22. Amy25508
23. Bcp12715
24. Ex-a1586
25. Zec63975
26. Lee011 Succinatelee011 Succinate
27. Hy-15777b
28. S5188
29. Ribociclib Succinate [who-dd]
30. Sb18482
31. Ac-30654
32. As-75241
33. Ribociclib Succinate [orange Book]
34. D10979
35. Lee-011 Succinate Salt, Ribociclib Succinate Salt
36. J-007026
37. Q27274660
38. Kisqali Femara Co-pack Component Ribociclib Succinate
39. Ribociclib Succinate Component Of Kisqali Femara Co-pack
40. 7-cyclopentyl-n,n-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7h-pyrrolo[2,3-d]pyrimidine-6-carboxamide; Butanedioic Acid
| Molecular Weight | 552.6 g/mol |
|---|---|
| Molecular Formula | C27H36N8O5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 552.28086628 g/mol |
| Monoisotopic Mass | 552.28086628 g/mol |
| Topological Polar Surface Area | 166 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 728 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | KISQALI |
| Active Ingredient | RIBOCICLIB SUCCINATE |
| Company | NOVARTIS PHARMS CORP (Application Number: N209092. Patents: 8324225, 8415355, 8685980, 8962630, 9193732, 9416136, 9868739) |
| 2 of 2 | |
|---|---|
| Drug Name | KISQALI FEMARA CO-PACK (COPACKAGED) |
| Active Ingredient | LETROZOLE; RIBOCICLIB SUCCINATE |
| Company | NOVARTIS PHARMS CORP (Application Number: N209935. Patents: 8324225, 8415355, 8685980, 8962630, 9193732, 9416136, 9868739) |
Kisqali is indicated for the treatment of women with hormone receptor (HR)positive, human epidermal growth factor receptor 2 (HER2)negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy.
In pre or perimenopausal women, the endocrine therapy should be combined with a luteinising hormonereleasing hormone (LHRH) agonist.
L01XE

