

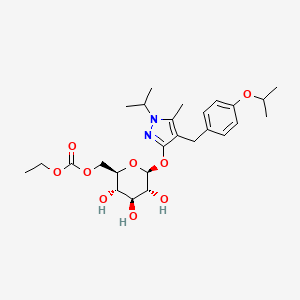

1. 4-(4-isopropoxybenzyl)-1-isopropyl-5-methyl-1h-pyrazol-3-yl 6-o-(ethoxycarbonyl)-beta-d-glucopyranoside
1. 442201-24-3
2. Gsk-189075
3. Gsk-189075a
4. Gsk189075a
5. Remogliflozin Etabonate [inn]
6. Tr0qt6qsul
7. Ethyl (((2r,3s,4s,5r,6s)-3,4,5-trihydroxy-6-((4-(4-isopropoxybenzyl)-1-isopropyl-5-methyl-1h-pyrazol-3-yl)oxy)tetrahydro-2h-pyran-2-yl)methyl) Carbonate
8. Ethyl [(2r,3s,4s,5r,6s)-3,4,5-trihydroxy-6-[5-methyl-1-propan-2-yl-4-[(4-propan-2-yloxyphenyl)methyl]pyrazol-3-yl]oxyoxan-2-yl]methyl Carbonate
9. 5-methyl-4-(4-(1-methylethoxy)benzyl)-1-(1-methylethyl)-1h-pyrazol-3-yl 6-o-(ethoxycarbonyl)-beta-d-glucopyranoside
10. Unii-tr0qt6qsul
11. Kgt-1681
12. Remogliflozin Etabonate [usan]
13. Beta-d-glucopyranoside, 5-methyl-4-((4-(1-methylethoxy)phenyl)methyl)-1-(1-methylethyl)-1h-pyrazol-3-yl, 6-(ethyl Carbonate)
14. Beta-d-glucopyranoside, 5-methyl-4-[[4-(1-methylethoxy)phenyl]methyl]-1-(1-methylethyl)-1h-pyrazol-3-yl, 6-(ethyl Carbonate)
15. S0993
16. Schembl721678
17. Chembl2028665
18. Dtxsid50963191
19. Chebi:177541
20. Gsk 189075a
21. Zinc3979756
22. Remogliflozin Etabonate (usan/inn)
23. Bdbm50559516
24. Gsk189075
25. Bhv-091009
26. Db12935
27. Remogliflozin Etabonate (gsk189075)
28. Remogliflozin Etabonate [who-dd]
29. Br162756
30. Hy-14945
31. Cs-0003650
32. D10055
33. Q7312052
34. 3-(6-o-ethoxycarbonyl-beta-d-glucopyranosyloxy)-4-[(4-isopropoxyphenyl)-methyl]-1-isopropyl-5-methylpyrazole
35. 3-(6-o-ethoxycarbonyl-beta-d-glucopyranosyloxy)-4-[(4-isopropoxyphenyl)methyl]-1-isopropyl-5-methylpyrazole
36. 4-(4-isopropoxybenzyl)-1-isopropyl-5-methyl-1h-pyrazole-3-yl 6-o-(ethoxycarbonyl)-beta-d-glucopyranoside
37. 5-methyl-1-(propan-2-yl)-4-((4-((propan-2-yl)oxy)phenyl)methyl)-1h-pyrazol-3-yl 6-o-(ethoxycarbonyl)-.beta.-d-glucopyranoside
38. 5-methyl-4-(4-(1-methylethoxy)benzyl)-1-(1-methylethyl)-1h-pyrazol-3-yl 6-o- (ethoxycarbonyl)-.beta.-d-glucopyranoside
| Molecular Weight | 522.6 g/mol |
|---|---|
| Molecular Formula | C26H38N2O9 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 522.25773079 g/mol |
| Monoisotopic Mass | 522.25773079 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 704 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sodium-Glucose Transporter 2 Inhibitors
Compounds that inhibit SODIUM-GLUCOSE TRANSPORTER 2. They lower blood sugar by preventing the reabsorption of glucose by the kidney and are used in the treatment of TYPE 2 DIABETES MELLITUS. (See all compounds classified as Sodium-Glucose Transporter 2 Inhibitors.)
