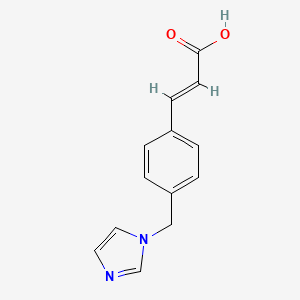

1. 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-2-propenoic Acid
2. 4-(1-imidazoylmethyl)cinnamic Acid
3. Oky 046
4. Oky-046
5. Ozagrel, Monohydrochloride
6. Ozagrel, Monohydrochloride, (e)-isomer
7. Sodium Ozagrel
1. 82571-53-7
2. Ozagrel [inn]
3. Oky-046
4. (e)-3-(4-((1h-imidazol-1-yl)methyl)phenyl)acrylic Acid
5. Ozagrel Free Acid
6. Kct-0809
7. Domenan
8. (e)-3-[4-(imidazol-1-ylmethyl)phenyl]prop-2-enoic Acid
9. Chembl11662
10. (e)-p-(imidazol-1-ylmethyl)cinnamic Acid
11. L256jb984d
12. 82571-53-7 (free Base)
13. Ozagrel (inn)
14. Ozagrelum [latin]
15. Pulmoza
16. Ozagrelum
17. 3-[4-(1h-imidazol-1-ylmethyl)phenyl]-2e-propenoic Acid
18. 2-propenoic Acid, 3-[4-(1h-imidazol-1-ylmethyl)phenyl]-, (2e)-
19. Unii-l256jb984d
20. Cataclot (tn)
21. Ozagrel,(s)
22. Ozagrel, Oky-046
23. Ozagrel [mi]
24. 2-propenoic Acid, 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-, (e)-
25. 3-[4-(1-imidazolylmethyl)phenyl]-2-propenoic Acid
26. Ozagrel [mart.]
27. Prestwick2_000979
28. Prestwick3_000979
29. Ozagrel [who-dd]
30. Schembl4210
31. Bspbio_001017
32. Bpbio1_001119
33. Gtpl9866
34. Oky046
35. Zinc5389
36. Dtxsid6048547
37. Chebi:92359
38. Chebi:134938
39. Hms2089o14
40. Hms3649m21
41. Hms3884n21
42. Bcp12147
43. Ex-a5744
44. Hy-b0428
45. Bdbm50017896
46. Mfcd00868231
47. S2496
48. Akos015889403
49. Ac-2080
50. Ccg-266782
51. Db12017
52. Ncgc00025195-02
53. As-71327
54. Ls-14195
55. Sbi-0207081.p001
56. Ab00514722
57. Sw197369-5
58. 3-(4-imidazol-1-ylmethyl-phenyl)-acrylic Acid
59. D08327
60. (e)-3-(4-((1h-imidazol-1-yl)methyl)phenyl)
61. Ab00514722-10
62. Ab00514722-12
63. Ab00514722-13
64. Ab00514722_14
65. Ab00514722_15
66. 4-(1h-imidazole-1-ylmethyl)benzenepropenoic Acid
67. 571o537
68. (e)-3-(4-imidazol-1-ylmethyl-phenyl)-acrylic Acid
69. Q-100843
70. Q7116436
71. Sr-01000597793-8
72. 3-[4-(imidazol-1-ylmethyl)phenyl]prop-2-enoic Acid
73. Brd-k19525698-003-01-1
74. Brd-k53061490-003-03-0
75. (e)-3-[4-(1h-imidiazol-1-ylmethyl)phenyl]-2-propenic Acid
76. (2e)-3-[4-(1h-imidazol-1-ylmethyl)phenyl]acrylic Acid
77. (2e)-3-{4-[(1h-imidazol-1-yl)methyl]phenyl}prop-2-enoic Acid
78. (e)-3-[p-(1h-imidazol-1-ylmethyl)phenyl]-2-propenoic Acid
| Molecular Weight | 228.25 g/mol |
|---|---|
| Molecular Formula | C13H12N2O2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 228.089877630 g/mol |
| Monoisotopic Mass | 228.089877630 g/mol |
| Topological Polar Surface Area | 55.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 283 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)