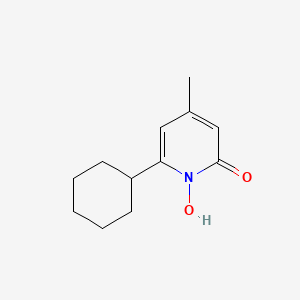

1. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Ethanolamine Salt
2. Batrafen
3. Ciclopirox Olamine
4. Ciclopiroxolamine
5. Cyclopirox
6. Cyclopyroxolamine
7. Dafnegin Csc
8. Dafnegin-csc
9. Hoe 296
10. Hoe-296
11. Hoe296
12. Loprox
13. Penlac
1. 29342-05-0
2. Loprox
3. Penlac
4. Hoe 296b
5. Cyclopirox
6. Ciclopiroxum
7. Hoe-296b
8. Ciclopiroxum [inn-latin]
9. 2(1h)-pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-
10. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one
11. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one
12. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone
13. Hoe296b
14. Ciclopirox (penlac)
15. Loprox (tn)
16. 19w019zdrj
17. Terit
18. Chebi:453011
19. 29342-05-0 (free)
20. Stieprox
21. Ciclopirox Gel
22. Loprox Cream
23. Loprox Gel
24. Ciclopirox Olamin
25. Ciclopirox-olamin
26. 6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one
27. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone
28. 6-cyclohexyl-1-hydroxy-4-methyl-1h-pyridin-2-one
29. Mls002153867
30. Penlac (tn)
31. Ciclopirox (usp/inn)
32. Cnl8
33. Smr001233223
34. Einecs 249-577-2
35. Unii-19w019zdrj
36. Ciclodan
37. Ciclopirox [usan:usp:inn:ban]
38. 2(1h)-pyridone, 6-cyclohexyl-1-hydroxy-4-methyl-
39. Ciclopirox [mi]
40. Ciclopirox [inn]
41. Prestwick0_000541
42. Prestwick1_000541
43. Prestwick2_000541
44. Prestwick3_000541
45. Spectrum2_000146
46. Spectrum3_000351
47. Spectrum4_000288
48. Spectrum5_000747
49. Ciclopirox [usan]
50. Ciclopirox [vandf]
51. 6-cyclohexyl-1-hydroxy-4-methyl-pyridin-2-one
52. Ciclopirox [mart.]
53. Chembl1413
54. Ciclopirox [usp-rs]
55. Ciclopirox [who-dd]
56. Schembl34424
57. Bspbio_000581
58. Bspbio_002041
59. Kbiogr_000816
60. 6-cyclohexyl-1-hydroxy-4-methyl-2-(1h)-pyridone
61. Cid_38911
62. Bidd:gt0080
63. (6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone)
64. Spbio_000252
65. Spbio_002502
66. Bpbio1_000641
67. Zinc1145
68. Ciclopirox [orange Book]
69. Ciclopirox, >=98% (hplc)
70. Dtxsid9048564
71. Bdbm66087
72. Gtpl11349
73. Kbio3_001261
74. Ciclopirox [ep Monograph]
75. Ciclopirox [usp Monograph]
76. Hms3656i12
77. Bcp28530
78. Hy-b0450
79. Mfcd00599441
80. S2528
81. Akos015895717
82. Ab06517
83. Ccg-266632
84. Db01188
85. Ks-5085
86. Ncgc00017112-04
87. Ncgc00017112-05
88. Ncgc00017112-06
89. Ncgc00017112-08
90. Ncgc00017112-11
91. Ncgc00178850-01
92. Ncgc00178850-02
93. Ac-24195
94. Ciclopirox 100 Microg/ml In Acetonitrile
95. Sbi-0206690.p002
96. Db-047566
97. Hoe 296; Hoe-296;ciclopiroxum; Penlac
98. 1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone
99. Ft-0602961
100. Sw196923-5
101. 6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone
102. D03488
103. Ab00053438_09
104. Ab00053438_10
105. Ab00053438_11
106. 342c050
107. A819878
108. Q419468
109. Sr-05000001589-5
110. W-106995
111. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone #
112. Brd-k13044802-213-04-1
113. Brd-k13044802-213-09-0
114. 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridone
115. 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one
116. Ciclopirox, European Pharmacopoeia (ep) Reference Standard
117. 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone
118. 2-azanylethanol;6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one
119. Ciclopirox, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 207.27 g/mol |
|---|---|
| Molecular Formula | C12H17NO2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 207.125928785 g/mol |
| Monoisotopic Mass | 207.125928785 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 325 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Ciclopirox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | Ciclopirox Topical Solution, 8% (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.Each gram of Ciclopirox Topical Solution, 8% (Nail Lacquer) con... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Gel; Shampoo; Cream; Solution; Suspension |
| Route | Topical |
| Strength | 8%; 1%; 0.77% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Glenmark Pharms; Hi Tech Pharma; Paddock; Versapharm; Actavis Mid Atlantic; Perrigo; Perrigo New York; Taro Pharm Inds; Taro; Tolmar; G And W Labs; Cipla |
| 2 of 6 | |
|---|---|
| Drug Name | Loprox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LOPROX (ciclopirox) Shampoo 1% contains the synthetic antifungal agent, ciclopirox for topical use.Each gram (equivalent to 0.96 mL) of LOPROX Shampoo contains 10 mg ciclopirox in a shampoo base consisting of disodium laureth sulfosuccinate, laureth-... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Shampoo; Cream; Suspension; Gel |
| Route | Topical |
| Strength | 1%; 0.77% |
| Market Status | Prescription |
| Company | Cnty Line Pharms; Medicis; Medimetriks Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Penlac |
| Drug Label | PENLAC NAIL LACQUER (ciclopirox) Topical Solution, 8%, contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin. Each gram of PENLAC NAIL LACQUER (ciclopirox) Topi... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 4 of 6 | |
|---|---|
| Drug Name | Ciclopirox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | Ciclopirox Topical Solution, 8% (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.Each gram of Ciclopirox Topical Solution, 8% (Nail Lacquer) con... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Gel; Shampoo; Cream; Solution; Suspension |
| Route | Topical |
| Strength | 8%; 1%; 0.77% |
| Market Status | Prescription |
| Company | Glenmark Generics; Fougera Pharms; Glenmark Pharms; Hi Tech Pharma; Paddock; Versapharm; Actavis Mid Atlantic; Perrigo; Perrigo New York; Taro Pharm Inds; Taro; Tolmar; G And W Labs; Cipla |
| 5 of 6 | |
|---|---|
| Drug Name | Loprox |
| PubMed Health | Ciclopirox (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LOPROX (ciclopirox) Shampoo 1% contains the synthetic antifungal agent, ciclopirox for topical use.Each gram (equivalent to 0.96 mL) of LOPROX Shampoo contains 10 mg ciclopirox in a shampoo base consisting of disodium laureth sulfosuccinate, laureth-... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Shampoo; Cream; Suspension; Gel |
| Route | Topical |
| Strength | 1%; 0.77% |
| Market Status | Prescription |
| Company | Cnty Line Pharms; Medicis; Medimetriks Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Penlac |
| Drug Label | PENLAC NAIL LACQUER (ciclopirox) Topical Solution, 8%, contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin. Each gram of PENLAC NAIL LACQUER (ciclopirox) Topi... |
| Active Ingredient | Ciclopirox |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
Used as a topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum.
FDA Label
Ciclopirox is a broad-spectrum antifungal medication that also has antibacterial and anti-inflammatory properties. Its main mode of action is thought to be its high affinity for trivalent cations, which inhibit essential co-factors in enzymes. Ciclopirox exhibits either fungistatic or fungicidal activity in vitro against a broad spectrum of fungal organisms, such as dermatophytes, yeasts, dimorphic fungi, eumycetes, and actinomycetes. In addition to its broad spectrum of action, ciclopirox also exerts antibacterial activity against many Gram-positive and Gram-negative bacteria. Furthermore, the anti-inflammatory effects of ciclopirox have been demonstrated in human polymorphonuclear cells, where ciclopirox has inhibited the synthesis of prostaglandin and leukotriene. Ciclopirox can also exhibit its anti-inflammatory effects by inhibiting the formation of 5-lipoxygenase and cyclooxygenase.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D01AE14
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE14 - Ciclopirox
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AX - Other antiinfectives and antiseptics
G01AX12 - Ciclopirox
Absorption
Rapidly absorbed after oral administration. Mean absorption of ciclopirox after application to nails of all twenty digits and adjacent 5 millimeters of skin once daily for 6 months in patients with dermatophytic onychomycoses was less than 5% of the applied dose. Ciclopirox olamine also penetrates into hair and through the epidermis and hair follicles into sebaceous glands and dermis.
Route of Elimination
Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides.
Glucuronidation is the main metabolic pathway of ciclopirox.
1.7 hours for 1% topical solution.
Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase. ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport.