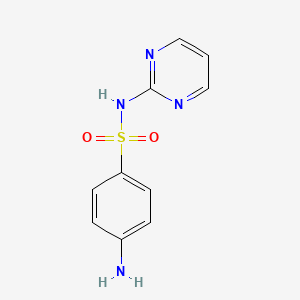

1. Sulfadiazine, Zinc
2. Sulfazin
3. Sulfazine
4. Sulphadiazine
5. Zinc Sulfadiazine
1. 68-35-9
2. Sulphadiazine
3. Sulfapyrimidine
4. Sulfadiazin
5. Adiazine
6. Sulfazine
7. Sulfadiazene
8. Adiazin
9. Debenal
10. Pyrimal
11. Liquadiazine
12. Sulfapyrimidin
13. 2-sulfanilamidopyrimidine
14. Coco-diazine
15. Cremodiazine
16. Spofadrizine
17. Theradiazine
18. Cremotres
19. Deltazina
20. Diazolone
21. Eskadiazine
22. Microsulfon
23. Neotrizine
24. Palatrize
25. Piridisir
26. Quadetts
27. Quadramoid
28. Sanodiazine
29. Sterazine
30. Sulfatryl
31. Sulfolex
32. Sulfonsol
33. Terfonyl
34. Trifonamide
35. Truozine
36. Diazin
37. Neazine
38. Pirimal
39. Sulfose
40. Trisem
41. Sulfanilamidopyrimidine
42. Honey Diazine
43. Lipo-levazine
44. Tri-sulfameth
45. Triple Sulfas
46. Lipo-diazine
47. Metha-meridiazine
48. Sulfadiazinum
49. Diazovit
50. Sulfadiazina
51. Sulphadiazine E
52. 4-amino-n-(pyrimidin-2-yl)benzenesulfonamide
53. Sulfapirimidin
54. Di-azo-mul
55. Thi-di-mer
56. 2-sulfanilylaminopyrimidine
57. Benzenesulfonamide, 4-amino-n-2-pyrimidinyl-
58. Codiazine
59. Silvadene
60. Pecta-diazine, Suspension
61. 4-amino-n-pyrimidin-2-ylbenzenesulfonamide
62. Rp 2616
63. Pyrimidine, 2-sulfanilamido-
64. 2-sulfapyrimidine
65. N(1)-2-pyrimidylsulfanilamide
66. N(1)-2-pyrimidinylsulfanilamide
67. 4-amino-n-2-pyrimidinylbenzenesulfonamide
68. N(sup 1)-2-pyrimidinylsulfanilamide
69. 4-amino-n-(pyrimidin-2-yl)benzene-1-sulfonamide
70. Sulfanilamide, N1-2-pyrimidinyl-
71. 4-amino-n-pyrimidin-2-yl-benzenesulfonamide
72. Chebi:9328
73. Sulfanilamide, N(sup 1)-2-pyrimidinyl-
74. 4-amino-n-2-pyrimidinyl-benzenesulfonamide
75. N(sup1)-2-pyrimidylsulfanilamide
76. Sulfanilamide, N1-2(1h)-pyrimidinylidene-
77. Silver Sulfadiazine
78. A-306
79. N(sup1)-2-pyrimidinylsulfanilamide
80. Chembl439
81. S. N. 112
82. Nsc35600
83. Nsc-35600
84. Cas-68-35-9
85. Ncgc00016305-01
86. Solfadiazina
87. Cocodiazine
88. Solfadiazina [dcit]
89. Sulfadiazine 100 Microg/ml In Acetonitrile
90. 0n7609k889
91. 2-sulfanilamidopyrimidin
92. Nsc117870
93. Sulfapyrimidin [german]
94. Sdz
95. Sulfadiazinum [inn-latin]
96. Sulfadiazina [inn-spanish]
97. Sildaflo
98. S.n. 112
99. 2-sulfanilamidopyrimidin [german]
100. A-306 (van)
101. 4-amino-n-(2-pyrimidinyl)benzenesulfonamide
102. A 306
103. Smr000059113
104. Sulfadiazine (tn)
105. N-(2-pyrimidinyl)sulfanilamide
106. Rbpi21 & Sulfa
107. Rp-2616
108. Sr-01000002973
109. Ssd
110. Einecs 200-685-8
111. Mfcd00006065
112. Nsc 35600
113. 2-sulfanilamido-pyrimidine
114. Brn 0235192
115. Diazine
116. Crl-8131 & Sulfadiazine
117. N1-2-pyrimidinylsulfanilamide
118. Sulfadiazine (jan/usp/inn)
119. Ai3-01047
120. Sulfadiazine,(s)
121. Trisulfapyrimidine, Oral Suspension
122. Unii-0n7609k889
123. Sulfadiazine [usp:inn:ban:jan]
124. Prestwick_428
125. Spectrum_000986
126. Sulfadiazina Reig Jofre
127. Sulfacombin (salt/mix)
128. Prestwick0_000023
129. Prestwick1_000023
130. Prestwick2_000023
131. Prestwick3_000023
132. Spectrum2_001319
133. Spectrum3_001362
134. Spectrum4_000342
135. Spectrum5_000992
136. Sulfadiazine [mi]
137. [(4-aminophenyl)sulfonyl]pyrimidin-2-ylamine
138. Sulfadiazine [inn]
139. Sulfadiazine [jan]
140. Epitope Id:140083
141. N1-2-pyrimidylsulfanilamide
142. Sulfadiazine, >=99.0%
143. Dsstox_cid_24130
144. Dsstox_rid_80105
145. Sulfadiazine [vandf]
146. Dsstox_gsid_44130
147. Oprea1_081078
148. Schembl24176
149. Bspbio_000085
150. Bspbio_002884
151. Kbiogr_000743
152. Kbioss_001466
153. Sulfadiazine [mart.]
154. 5-25-10-00067 (beilstein Handbook Reference)
155. Mls000069423
156. Mls006011457
157. Divk1c_000543
158. Spectrum1500546
159. Sulfadiazine [usp-rs]
160. Sulfadiazine [who-dd]
161. Spbio_001417
162. Spbio_002006
163. Bpbio1_000095
164. Wln: T6n Cnj Bmswr Dz
165. Dtxsid7044130
166. Hms501l05
167. Kbio1_000543
168. Kbio2_001466
169. Kbio2_004034
170. Kbio2_006602
171. Kbio3_002104
172. Sulfadiazine [green Book]
173. Ninds_000543
174. Glxc-25873
175. Hms1568e07
176. Hms1921a13
177. Hms2090p09
178. Hms2092i15
179. Hms2095e07
180. Hms2235d19
181. Hms3371l19
182. Hms3655i10
183. Hms3712e07
184. Pharmakon1600-01500546
185. Sulfadiazine [orange Book]
186. Zinc120319
187. Sulfadiazine [ep Monograph]
188. Sulfadiazine [usp Impurity]
189. Albb-014888
190. Amy33423
191. Bcp12140
192. Hy-b0273
193. Sulfadiazine [usp Monograph]
194. Tox21_110360
195. Bbl013169
196. Bdbm50166571
197. Ccg-39257
198. Nsc757324
199. Recombinant Bactericidal/permeability-increasing Protein & Sulfadiazine
200. S1770
201. Stk317797
202. Sulfose Component Sulfadiazine
203. Terfonyl Component Sulfadiazine
204. 2-(p-aminobenzenesulfonamido)pyrimidin
205. Akos000119073
206. Lantrisul Component Sulfadiazine
207. Sulfaloid Component Sulfadiazine
208. Db00359
209. Ks-1144
210. Nsc-757324
211. Sulfadiazin 100 Microg/ml In Methanol
212. 2-(4-aminobenzenesulfonamido)pyrimidine
213. 4-amino-n-2-pyrimidylbenzenesulfonamide
214. Idi1_000543
215. Neotrizine Component Sulfadiazine
216. Sulfadiazine Component Of Sulfose
217. Ncgc00016305-02
218. Ncgc00016305-03
219. Ncgc00016305-04
220. Ncgc00016305-05
221. Ncgc00016305-06
222. Ncgc00016305-09
223. Ncgc00016305-10
224. Ncgc00016305-11
225. Ncgc00023291-03
226. Ncgc00023291-04
227. Sulfadiazine (trisulfapyrimidines)
228. Sulfadiazine Component Of Terfonyl
229. Trisulfapyrimidines (sulfadiazine)
230. 2-(4-aminobenzenesulfonylamino)pyrimidine
231. Ac-26817
232. Sulfadiazine 1000 Microg/ml In Methanol
233. Sulfadiazine Component Of Lantrisul
234. Sulfadiazine Component Of Sulfaloid
235. 4-amino-n-2-pyrimidinyl Benzenesulfonamide
236. Mixture Of Sulfadiazine, And Sulfamethazine
237. Sbi-0051520.p003
238. Sulfadiazine Component Of Neotrizine
239. Triple Sulfoid Component Sulfadiazine
240. 4-[[(pyrimidin-2-yl)amino]sulfonyl]aniline
241. Ab00052095
242. Benzenesulfonamide,4-amino-n-2-pyrimidinyl-
243. Ft-0674739
244. Ft-0674740
245. Ft-0674741
246. S0579
247. Sulfadiazine For Identification Of Impurity F
248. Sw196657-3
249. 4-amino-n-(2-pyrimidinyl) Benzenesulfonamide
250. Sulfadimidine Impurity B [ep Impurity]
251. 4-amino-n-(2-pyrimidinyl)benzenesulfonamide #
252. 68s359
253. C07658
254. D00587
255. D92267
256. Sulfadiazine Component Of Triple Sulfoid
257. Trisulfapyrimidine, Oral Suspension (salt/mix)
258. Ab00052095-13
259. Ab00052095-14
260. Ab00052095_15
261. Ab00052095_16
262. Benzenesulfonamide, 4-amino-n-(2-pyrimidinyl)-
263. Sulfadiazine, Vetranal(tm), Analytical Standard
264. Sulfonamides Duplex Component Sulfadiazine
265. A836115
266. Q-201759
267. Q2555060
268. Sr-01000002973-2
269. Sr-01000002973-3
270. Sulfadiazine Component Of Sulfonamides Duplex
271. Brd-k32273377-001-05-4
272. Brd-k32273377-001-09-6
273. F1657-1720
274. Sulfadiazine, Certified Reference Material, Tracecert(r)
275. Trisulfapyrimidines (sulfadiazine) [orange Book]
276. Z271004844
277. Sulfadiazine, European Pharmacopoeia (ep) Reference Standard
278. Sulfadiazine, United States Pharmacopeia (usp) Reference Standard
279. 4-amino-n-(2-pyrimidinyl)benzenesulfonamide, N1-(pyrimidin-2-yl)sulfanilamide
280. Sulfadiazine, Pharmaceutical Secondary Standard; Certified Reference Material
281. 141582-64-1
282. Sulfadiazine For Identification Of Impurity F, European Pharmacopoeia (ep) Reference Standard
1. Sulfadiazine Sodium
2. Sodium Sulfadiazine
3. Sodium Sulfapyrimidine
| Molecular Weight | 250.28 g/mol |
|---|---|
| Molecular Formula | C10H10N4O2S |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 250.05244675 g/mol |
| Monoisotopic Mass | 250.05244675 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Silvadene |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | King Pharms |
| 2 of 8 | |
|---|---|
| Drug Name | Ssd |
| PubMed Health | Sulfadiazine (By mouth) |
| Drug Classes | Antibiotic |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 3 of 8 | |
|---|---|
| Drug Name | Sulfadiazine |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Sulfadiazine is an oral sulfonamide anti-bacterial agent.Each tablet, for oral administration, contains 500 mg sulfadiazine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, docusate sodium, microcrystallin... |
| Active Ingredient | Sulfadiazine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Sandoz |
| 4 of 8 | |
|---|---|
| Drug Name | Thermazene |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Thepharmanetwork |
| 5 of 8 | |
|---|---|
| Drug Name | Silvadene |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | King Pharms |
| 6 of 8 | |
|---|---|
| Drug Name | Ssd |
| PubMed Health | Sulfadiazine (By mouth) |
| Drug Classes | Antibiotic |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 7 of 8 | |
|---|---|
| Drug Name | Sulfadiazine |
| PubMed Health | Silver Sulfadiazine (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Sulfadiazine is an oral sulfonamide anti-bacterial agent.Each tablet, for oral administration, contains 500 mg sulfadiazine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, docusate sodium, microcrystallin... |
| Active Ingredient | Sulfadiazine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Sandoz |
| 8 of 8 | |
|---|---|
| Drug Name | Thermazene |
| Active Ingredient | Silver sulfadiazine |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Thepharmanetwork |
For the treatment of rheumatic fever and meningococcal meningitis
Sulfadiazine is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Coccidiostats
Agents useful in the treatment or prevention of COCCIDIOSIS in man or animals. (See all compounds classified as Coccidiostats.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
J01EC02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J01EC02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BA - Sulfonamides
D06BA01 - Silver sulfadiazine
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EC - Intermediate-acting sulfonamides
J01EC02 - Sulfadiazine
Route of Elimination
Sulfadiazine is excreted largely in the urine.
Sulfadiazine has known human metabolites that include Sulfadiazine hydroxylamine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Sulfadiazine is a competitive inhibitor of the bacterial enzyme dihydropteroate synthetase. This enzyme is needed for the proper processing of para-aminobenzoic acid (PABA) which is essential for folic acid synthesis. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.