


1. Biltricide
2. Cesol
3. Cisticid
4. Cysticide
5. Droncit
6. Drontsit
7. Embay 8440
8. Prasiquantel
9. Praziquantel, (+-)-isomer
10. Praziquantel, (r)-isomer
11. Praziquantel, (s)-isomer
12. Pyquiton
13. Traziquantel
1. 55268-74-1
2. Biltricide
3. Droncit
4. Pyquiton
5. Cesol
6. Embay 8440
7. Praziquantelum
8. Azinox
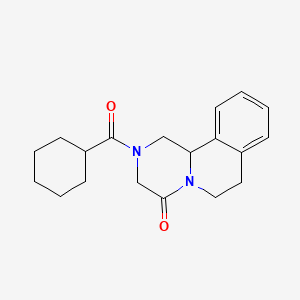
9. 2-(cyclohexanecarbonyl)-3,6,7,11b-tetrahydro-1h-pyrazino[2,1-a]isoquinolin-4-one
10. 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4h-pyrazino[2,1-a]isoquinolin-4-one
11. Embay-8440
12. Mls000038419
13. Praziquantel (biltricide)
14. Mfcd00058531
15. 135526-78-2
16. Nsc-757285
17. Smr000037139
18. 2-(cyclohexanecarbonyl)-1,2,3,6,7,11b-hexahydro-4h-pyrazino[2,1-a]isoquinolin-4-one
19. 2-(cyclohexanecarbonyl)-2,3,6,7-tetrahydro-1h-pyrazino[2,1-a]isoquinolin-4(11bh)-one
20. 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4h-pyrazino(2,1-a)isoquinolin-4-one
21. 4h-pyrazino(2,1-a)isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-
22. Cysticide
23. Cutter Tape Tabs
24. 2-cyclohexanecarbonyl-1h,2h,3h,4h,6h,7h,11bh-piperazino[2,1-a]isoquinolin-4-one
25. Distocide
26. Prazinon
27. 6490c9u457
28. Dsstox_cid_1182
29. Dsstox_rid_75995
30. Dsstox_gsid_21182
31. Praziquantelum [inn-latin]
32. Cutter
33. 4h-pyrazino[2,1-a]isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-
34. Biltricide (tn)
35. Ccris 4114
36. (+/-)-praziquantel
37. Sr-01000003100
38. Einecs 259-559-6
39. Brn 0761557
40. Bay-8440
41. Praziquantel (jan/usp/inn)
42. Praziquantel,(s)
43. Unii-6490c9u457
44. Ncgc00016877-01
45. Prestwick_402
46. Cas-55268-74-1
47. Praziquantel 100 Microg/ml In Acetonitrile
48. (+-)-praziquantel
49. Praziquantel [usan:usp:inn:ban:jan]
50. Spectrum_001119
51. Opera_id_378
52. Prestwick0_000260
53. Prestwick1_000260
54. Prestwick2_000260
55. Prestwick3_000260
56. Spectrum2_001288
57. Spectrum3_000550
58. Spectrum4_000482
59. Spectrum5_001064
60. Praziquantel [mi]
61. Praziquantel [inn]
62. Praziquantel [jan]
63. Chembl976
64. P 4668
65. Praziquantel [usan]
66. Cid_4891
67. Praziquantel [vandf]
68. Lopac0_000909
69. Oprea1_163497
70. Schembl44153
71. Bspbio_000080
72. Bspbio_002199
73. Kbiogr_000963
74. Kbioss_001599
75. Praziquantel [mart.]
76. 5-24-03-00361 (beilstein Handbook Reference)
77. Mls000028528
78. Mls001201812
79. Mls001304085
80. Mls002548849
81. Mls006011880
82. Divk1c_000130
83. Praziquantel [usp-rs]
84. Praziquantel [who-dd]
85. Praziquantel [who-ip]
86. Spectrum1500494
87. Spbio_001295
88. Spbio_002299
89. Praziquantel-(cyclohexyl-d11)
90. Bpbio1_000088
91. Dtxsid9021182
92. Schembl16019896
93. Bdbm74574
94. Chebi:91583
95. Hms500g12
96. Kbio1_000130
97. Kbio2_001599
98. Kbio2_004167
99. Kbio2_006735
100. Kbio3_001699
101. Praziquantel [green Book]
102. Ninds_000130
103. Glxc-26256
104. Hms1568d22
105. Hms1920j06
106. Hms2090j19
107. Hms2092a09
108. Hms2095d22
109. Hms3259k07
110. Hms3262f20
111. Hms3655o19
112. Hms3712d22
113. Pharmakon1600-01500494
114. Praziquantel [orange Book]
115. Praziquantel For System Suitability
116. Praziquantel [ep Monograph]
117. Praziquantel [usp Impurity]
118. Amy16524
119. Bcp17829
120. Bcp28525
121. Bcp30228
122. Hy-b0244
123. Wzb34336
124. Praziquantel [usp Monograph]
125. Tox21_110660
126. Tox21_201950
127. Tox21_302927
128. Tox21_500909
129. Ccg-39773
130. Nsc757285
131. Praziquantel, Anthelminic, Neurogenic
132. S1691
133. Stk030186
134. Praziquantelum [who-ip Latin]
135. Akos000541869
136. Akos016398525
137. Broadline Component Praziquantel
138. Profender Component Praziquantel
139. Tox21_110660_1
140. Ac-8426
141. Db01058
142. Lp00909
143. Nc00468
144. Nsc 757285
145. Sb49202
146. Sdccgsbi-0050884.p005
147. 2-(cyclohexylcarbonyl)-1,2,3,6,7-11b-hexahydro-4h-pyrazinoe(2,1a)isoquinolin-4-one
148. Idi1_000130
149. Ncgc00015818-04
150. Ncgc00015818-05
151. Ncgc00015818-06
152. Ncgc00015818-07
153. Ncgc00015818-08
154. Ncgc00015818-11
155. Ncgc00015818-12
156. Ncgc00015818-14
157. Ncgc00015818-15
158. Ncgc00015818-26
159. Ncgc00089733-02
160. Ncgc00089733-03
161. Ncgc00089733-04
162. Ncgc00089733-05
163. Ncgc00256422-01
164. Ncgc00259499-01
165. Ncgc00261594-01
166. As-12459
167. Bp166192
168. Praziquantel Component Of Broadline
169. Praziquantel Component Of Profender
170. Sy052322
171. Sbi-0050884.p004
172. Db-052707
173. Praziquantel (ema Epar: Veterinary)
174. (s)-praziquantel; Praziquantel, (s)-isomer
175. Ab00052075
176. Eu-0100909
177. Ft-0630676
178. P2125
179. Sw196645-3
180. 1h-pyrazino[2,1-a]isoquinolin-4(11bh)-one
181. 2-(cyclohexanecarbonyl)-2,3,6,7-tetrahydro-
182. C07367
183. D00471
184. Ab00052075-13
185. Ab00052075-15
186. Ab00052075_16
187. Ab00052075_17
188. Praziquantel, Vetranal(tm), Analytical Standard
189. 268p741
190. A830562
191. Q424145
192. Praziquantel, Antibiotic For Culture Media Use Only
193. Q-201612
194. Sr-01000003100-3
195. Sr-01000003100-5
196. Sr-01000003100-7
197. Brd-a21858158-001-05-2
198. Brd-a21858158-001-16-9
199. Sr-01000003100-16
200. F0037-0136
201. Z1563145961
202. Praziquantel, European Pharmacopoeia (ep) Reference Standard
203. Praziquantel, United States Pharmacopeia (usp) Reference Standard
204. 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro- 4h-pyrazino[2,1-a]-isoquinolin-4-one
205. 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4h-pyrazino-[2,1- A]isoquinolin-4-one
206. 2-[cyclohexyl(oxo)methyl]-3,6,7,11b-tetrahydro-1h-pyrazino[2,1-a]isoquinolin-4-one
207. 2-cyclohexylcarbonyl-1,2,3,6,7,11b-hexahydro-4h-pyrazino(2,1-a)isoquinolin-4-one
208. Praziquantel For System Suitability, European Pharmacopoeia (ep) Reference Standard
209. Praziquantel, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 312.4 g/mol |
|---|---|
| Molecular Formula | C19H24N2O2 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 312.183778013 g/mol |
| Monoisotopic Mass | 312.183778013 g/mol |
| Topological Polar Surface Area | 40.6 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 472 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Biltricide |
| PubMed Health | Praziquantel (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | BILTRICIDE (praziquantel) is a trematodicide provided in tablet form for the oral treatment of schistosome infections and infections due to liver fluke.BILTRICIDE (praziquantel) is 2-(cyclohexylcarbonyl)-1,2,3,6,7, 11b-hexahydro-4H-pyrazino [2, 1... |
| Active Ingredient | Praziquantel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Biltricide |
| PubMed Health | Praziquantel (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | BILTRICIDE (praziquantel) is a trematodicide provided in tablet form for the oral treatment of schistosome infections and infections due to liver fluke.BILTRICIDE (praziquantel) is 2-(cyclohexylcarbonyl)-1,2,3,6,7, 11b-hexahydro-4H-pyrazino [2, 1... |
| Active Ingredient | Praziquantel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
For the treatment of infections due to all species of schistosoma.
FDA Label
Praziquantel is an anthelmintic used in most schistosome and many cestode infestations. Praziquantel effects the permeability of the cell membrane resulting in the contraction of schistosomes. The drug further causes vacuolization and disintegration of the schistosome tegument. The effect is more marked on adult worms compared to young worms. An increased calcium influx may play an important role. Secondary effects are inhibition of glucose uptake, lowering of glycogen levels and stimulation of lactate release. The action of praziquantel is limited very specifically to trematodes and cestodes; nematodes (including filariae) are not affected.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02B - Antitrematodals
P02BA - Quinoline derivatives and related substances
P02BA01 - Praziquantel
Absorption
Rapidly absorbed (80%)
renal
0.8-1.5 hours (in serum)
Praziquantel works by causing severe spasms and paralysis of the worms' muscles. This paralysis is accompanied - and probably caused - by a rapid Ca 2+ influx inside the schistosome. Morphological alterations are another early effect of praziquantel. These morphological alterations are accompanied by an increased exposure of schistosome antigens at the parasite surface. The worms are then either completely destroyed in the intestine or passed in the stool. An interesting quirk of praziquantel is that it is relatively ineffective against juvenile schistosomes. While initially effective, effectiveness against schistosomes decreases until it reaches a minimum at 3-4 weeks. Effectiveness then increases again until it is once again fully effective at 6-7 weeks. Glutathione S-transferase (GST), an essential detoxification enzyme in parasitic helminths, is a major vaccine target and a drug target against schistosomiasis. Schistosome calcium ion channels are currently the only known target of praziquantel.
