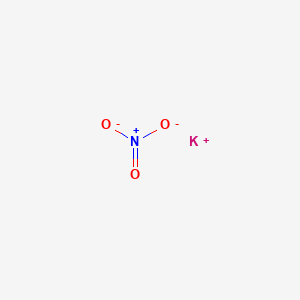

1. Potassium Nitrate Monohydrate
2. Saltpeter
3. Saltpetre
1. Saltpeter
2. 7757-79-1
3. Nitre
4. Saltpetre
5. Nitrate Of Potash
6. Nitric Acid Potassium Salt
7. Potassium;nitrate
8. Nitric Acid, Potassium Salt
9. Kaliumnitrat
10. Kali Nitricum
11. Kno3
12. Mfcd00011409
13. Ru45x2jn0z
14. Ins No.252
15. Vicknite
16. Chebi:63043
17. Ins-252
18. Nsc-57632
19. Kalii Nitras
20. E-252
21. Dsstox_cid_9692
22. Dsstox_rid_78811
23. Dsstox_gsid_29692
24. Salt Peter (van)
25. Chembl1644029
26. Caswell No. 697
27. Kaliumnitrat [german]
28. Potassium Nitrate(dot)
29. Potassium Nitrate [jan]
30. Cas-7757-79-1
31. Ccris 3667
32. Hsdb 1227
33. Einecs 231-818-8
34. Nsc 57632
35. Un1486
36. Unii-ru45x2jn0z
37. Epa Pesticide Chemical Code 076103
38. Potassium Nitrate [usp:jan]
39. Ai3-51245
40. Potassium-nitrate
41. Sensodyne (tn)
42. Nitric Acid Potassium Salt (1:1)
43. Potassium Nitrate,(s)
44. Potassium Nitrate (kno3)
45. Ec 231-818-8
46. Potassium Nitrate Acs Grade
47. Kali Nitricum [hpus]
48. Potassium Nitrate (jan/usp)
49. Potassium Nitrate [mi]
50. Potassium Nitrate [fcc]
51. Dtxsid4029692
52. Potassium Nitrate [hsdb]
53. Potassium Nitrate, P.a., 99%
54. Potassium Nitrate [vandf]
55. Potassium Nitrate, Puratronic(r)
56. Potassium Nitrate [mart.]
57. Potassium Nitrate [usp-rs]
58. Potassium Nitrate [who-dd]
59. Tox21_201581
60. Tox21_303394
61. Potassium Nitrate, Ar, >=99.5%
62. Potassium Nitrate, Lr, >=99.5%
63. Akos015902862
64. Akos024418772
65. Potassium Nitrate, Cell Culture Tested
66. Db11090
67. Potassium Nitrate [ep Impurity]
68. Potassium Nitrate [ep Monograph]
69. Potassium Nitrate, Bioxtra, >=99.0%
70. Ncgc00249235-01
71. Ncgc00257274-01
72. Ncgc00259130-01
73. Potassium Nitrate [usp Monograph]
74. Bp-31027
75. E252
76. Potassium Nitrate [un1486] [oxidizer]
77. Potassium Nitrate, Acs Reagent, >=99.0%
78. Potassium Nitrate, Nist(r) Srm(r) 193
79. Ft-0698960
80. Potassium Nitrate, Bioultra, >=99.5% (t)
81. D02051
82. Potassium Nitrate, 99.99% Trace Metals Basis
83. Potassium Nitrate, Reagentplus(r), >=99.0%
84. Potassium Nitrate, Saj First Grade, >=99.0%
85. Potassium Nitrate, Trace Metals Grade 99.99%
86. Potassium Nitrate, 99.999% Trace Metals Basis
87. Potassium Nitrate, Jis Special Grade, >=99.0%
88. Nitrate Nitrogen Standard Solution, 100 Ppm No3-
89. Q177836
90. Potassium Nitrate, Anhydrous, 99.99% Trace Metals Basis
91. Nitrogen And Oxygen Isotopes In Nitrate, Nist(r) Rm 8568
92. Potassium Standard For Aas, Ready-to-use, In Nitric Acid
93. Potassium Standard For Icp, For Icp, Ready-to-use, In Nitric Acid
94. Usgs32 (nitrogen And Oxygen Isotopes In Nitrate), Nist Rm 8558
95. Nitrate Ion Standard Solution, 0.01 M No3-, For Ion-selective Electrodes
96. Potassium Nitrate, United States Pharmacopeia (usp) Reference Standard
97. Potassium Nitrate, Bioreagent, Suitable For Cell Culture, Suitable For Plant Cell Culture
98. Potassium Nitrate, Puriss. P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., >=99%
99. Mettler-toledo Calibration Substance Me 51143095, Potassium Nitrate, Traceable To Primary Standards (lgc)
100. Potassium Nitrate, Puriss., Meets Analytical Specification Of Ph??eur, Bp, Usp, Fcc, E252, 99.0-100.5%
| Molecular Weight | 101.103 g/mol |
|---|---|
| Molecular Formula | KNO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 100.95152435 g/mol |
| Monoisotopic Mass | 100.95152435 g/mol |
| Topological Polar Surface Area | 62.9 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Toothpastes intended to prevent caries and to reduce painful sensitivity of the teeth are regulated as over-the-counter (OTC) anticaries drug products at Title 21, Code of Federal Regulations (21 CFR), Part 355. Such products may contain up to 5% potassium nitrate as a tooth desensitizing ingredient.
57 FR 20 114 (May 11, 1992)
Dentinal hypersensitivity occurs when gingival recession exposes dentin at the cervical margins of teeth. Twenty-four periodontal patients, with postoperative hypersensitive dentin were treated by burnishing saturated potassium nitrate (KNO3) to relieve pain. Using a visual analogue scale with participants acting as their own control, a subjective assessment of pain was measured and compared before and after KNO3 application. Thirty-six regions involving 98 teeth were assessed. A significant reduction of sensitivity and pain was achieved by using a saturated KNO3 solution (p < .0001 Student-t).
PMID:10321150 Touyz LZ, Stern J; .Gen Dent 47 (1): 42-5 (1999)
... Potassium nitrate has been used ... in a dentifrice or gel to alleviate dentinal hypersensitivity. The aim of this study was to compare a 3% potassium nitrate/0.2% sodium fluoride mouthwash with a 0.2% sodium fluoride control mouthwash in a 6-week double-blind study. ... Fifty subjects were evaluated using 2 tactile methods and cold air sensitivity (dental air syringe), along with subjective perception of pain (0 to 10 scale) at baseline and at 2 and 6 weeks. ... There was a general decrease in dentinal hypersensitivity levels in both groups over the 6-week study period as demonstrated by all 4 methods of assessment. There was also a statistically significant difference in decrease in sensitivity between the groups. /The authors concluded that/ this study showed that a 3% potassium nitrate/0.2% sodium fluoride mouthwash appears to have therapeutic potential to alleviate dentinal hypersensitivity.
PMID:118115 Pereira R. Chava VK; J Periodontol 72 (12): 1720-5 (2001)
The effect on dentinal hypersensitivity from the use of a new dentifrice containing 5.0% potassium nitrate and 0.454% stannous fluoride in a silica base (Colgate Sensitive Maximum Strength Toothpaste, Colgate-Palmolive Co.) over an 8-week period was compared to a commercially available dentifrice containing 5.0% potassium nitrate and 0.243% sodium fluoride in a silica base (positive control (Sensodyne Fresh Mint Toothpaste, Block Drug Company, Inc.)) and to a commercially available nondesensitizing dentifrice containing 0.243% sodium fluoride in a silica base (negative control (Colgate Winterfresh Gel, Colgate-Palmolive Co.)). A total of 120 participants were stratified into 3 balanced groups according to baseline mean air blast (thermal) and tactile (Yeaple Probe) sensitivity scores, gender, and age. Participants brushed their teeth twice daily (morning and evening) for 1 minute. Dentinal hypersensitivity examinations were conducted at baseline, 4 weeks, and 8 weeks by the same dental examiner. After 4- and 8-weeks' use of their assigned products, participants in the new dentifrice group demonstrated statistically significant improvements (p < 0.05) in tactile and air blast sensitivity, as compared to those using the positive and negative control dentifrices.
PMID:11908358 Schiff T et al; Compend Contin Educ Dent Suppl (27): 4-10 (2000)
For more Therapeutic Uses (Complete) data for POTASSIUM NITRATE (10 total), please visit the HSDB record page.
VET: AVOID USE IN PRESENCE OF SEVERE RENAL IMPAIRMENT.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 476
VET: POTASSIUM SALTS HAVE BEEN USED IN PAST AS DIURETICS BUT THEY ARE POTENTIALLY DANGEROUS & ... USE IS DISCOURAGED. /POTASSIUM SALTS/
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 576
METHEMOGLOBINEMIA HAS BEEN NOTED AFTER OVERDOSE OF POTASSIUM NITRATE.
FAIVRE J ET AL; ANN NUTR ALIMENT 30 (5-6): 8 831 (1976)
The toxic dose varies greatly; from 15 to 30 g /KNO3/ may prove fatal but much larger doses have been taken without serious effects.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1746
The lethal oral dose of potassium nitrate for an adult has been estimated to be between 4 and 30 g (about 40 to 300 mg NO3-/kg).
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
Fatal dose for adult humans is given as 30 to 35 g KNO3 ...
European Chemicals Bureau; IUCLID Dataset, Potassium nitrate (7757-79-1) (2000 CD-ROM edition). Available from, as of October 25, 2006: https://esis.jrc.ec.europa.eu/
For the relief of tooth sensitivity, and is also used as a pesticide, insecticide, as a food additive, and a rodenticide.
The potassium cation is an essential electrolyte that is important for the maintenance of intracellular osmotic pressure and for the maintenance of cell membrane potential, in particular, the potential of electrically excitable tissues. It is a regular component of the diet and is particularly abundant in fruit and vegetables. The recommended daily intake varies from 350-1275 mg in children to 1875 and 5625 mg in adults. In the United Kingdom, the recommended intake is 3.5 g/day for healthy adults. Potassium ions are believed to disturb the synapse between nerve cells, thus decreasing nerve excitation and the associated pain. Potassium nitrates are ignitable fumigants also utilized as rodenticides and insecticides. They are added to other pesticide active ingredients (sulfur and carbon) and placed into fumigant gas cartridges, designed to be ignited and placed in pest-infested areas. The activated cartridge bombs release toxic gases which are lethal to select rodents, skunks, coyotes, and wasps. Potassium ions have demonstrated in animal studies to act directly on the nerves and to reduce sensory activity. Tooth hypersensitivity can be relieved by inactivating the intra-dental nerve and inhibiting neural transmission, using suitable medications. It has been found that potassium-to-sodium intake ratios are strongly related to cardiovascular disease risk than either nutrient alone. The data describing this relationship warrants further research for various target tissue endpoints.
Explosive Agents
Substances that are energetically unstable and can produce a sudden expansion of the material, called an explosion, which is accompanied by heat, pressure and noise. Other things which have been described as explosive that are not included here are explosive action of laser heating, human performance, sudden epidemiological outbreaks, or fast cell growth. (See all compounds classified as Explosive Agents.)
Absorption
It is established that nitrate is quickly and almost entirely absorbed from the proximal and small intestine subsequent to ingestion in most animals, with little if any absorption from the stomach and lower intestine. The vast majority of intestinal K+ absorption occurs in the small intestine; the contribution of the normal colon to net K+ absorption and secretion is trivial.
Route of Elimination
Nitrates are excreted in the urine primarily as inorganic nitrates.
Volume of Distribution
Nitrates are absorbed into the general blood circulation and are transported across the body. Radioactive tracer experiments have demonstrated that nitrates are distributed evenly among body organs, and their rate of distribution depends on blood flow.
It is generally assumed that absorption takes place in upper portion of small intestine & ... excretion is primarily, if not exclusively, through kidney. ... preliminary observations ... have shown that not all animals reduce nitrate to nitrite in saliva. It is of considerable significance that major differences occur among mammalian species in the ability to concn nitrate from plasma into saliva. Large interspecies differences have also been shown to occur in elimination kinetics of nitrate. /Nitrate/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 415
Nitrate and nitrite given orally are absorbed and transferred to the blood in the upper part of the gastrointestinal tract. Abundant pectin in the food may delay absorption which may then occur lower down in the intestine, with possible increased risk for microbial transformation of nitrate into nitrite. /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
Regardless of route of exposure, nitrate and nitrite are rapidly transferred into the blood. Nitrite is gradually oxidized to nitrate which is readily distributed into most body fluids (urine, saliva, gastric juice, sweat, ileostomy fluid). Distribution of nitrate into plasma, erythrocytes, saliva and urine following an oral dose of sodium nitrate has been demonstrated ... Nitrate does not accumulate in the body. /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
Approximately 60% of oral nitrate is excreted in urine ... bacterial or endogenous metabolism probably accounts for the remainder. A minor part is excreted in sweat. /Nitrate/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
... Potassium nitrate ... /is/ rapidly absorbed and excreted unchanged ... Under some circumstances, however, appreciable amt of nitrate are converted to nitrite.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-316
Nitrates are reduced to nitrites by the bacteria in saliva and the gastrointestinal system. The in vivo reduction of nitrates to nitrites depends on conditions that are subject to much variations such the volume and species of microflora present in the saliva/gastrointestinal tract, and stomach pH. Gastric pH is higher in infants younger than 6 months of age and during certain gastrointestinal tract infections, thereby favoring the reduction of nitrates. Nitrate is metabolized to a small extent. The biotransformation of potassium nitrate consists of nitrate reduction, nitrite formation, nitrite reoxidation to nitrate, and formation of methemoglobin or NO, in a dynamic equilibrium,,.
Nitrate salts/ including potassium nitrate/ ... if not promptly absorbed, they may be reduced to nitrites by bacteria in bowel. I
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-124
... nitrate metabolism in man cannot be readily predicted from animal data. Several studies have suggested that large differences in nitrate metab may occur between individuals. These differences can span about three orders of magnitude when all available data, incl diet & physiological status, are taken into consideration. /nitrate/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 415
Where bacteria are present and the environment can be anerobic, nitrate can be reduced to nitrite. The main site for this reaction is mouth and stomach, but nitrite formation in the lower intestine and in the bladder (urinary infection) may also be of some toxicological importance. Nitrite may be further reduced to nitrogen by bacteria under some conditions. In blood, nitrite transforms hemoglobin to methemoglobin and is simultaneously oxidized to nitrate. Normally methemoglobin gradually reverts to hemoglobin through enzymatic reactions. Nitrite has vasodilating properties, probably through transformation into nitric oxide (NO) or a NO-containing molecule acting as a signal factor for smooth muscle relaxation. Nitrite easily transforms into a nitrosating agent in an acidic environment and can react with a variety of compounds, eg ascorbic acid, amines, amides. Nitrosation can also be mediated by bacteria, eg in the stomach. Some reaction products are carcinogenic (eg most nitrosoamines and amides). /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
BACKGROUND/AIMS: It has been suggested that dietary nitrate, after concentration in the saliva and reduction to nitrite by tongue surface bacteria, is chemically reduced to nitric oxide (NO) in the acidic conditions of the stomach. This study aimed to quantify this in humans. METHODS: Ten healthy fasting volunteers were studied twice, after oral administration of 2 mmol of potassium nitrate or potassium chloride. Plasma, salivary and gastric nitrate, salivary and gastric nitrite, and gastric headspace NO concentrations were measured over six hours. RESULTS: On the control day the parameters measured varied little from basal values. Gastric nitrate concentration was 105.3+/-13 umol/L (mean (SEM), plasma nitrate concentration was 17.9+/-2.4 umol/L, salivary nitrate concentration 92.6+/-31.6 umol/L, and nitrite concentration 53.9+/-22.8 umol/L. Gastric nitrite concentrations were minimal (< 1 mumol/l). Gastric headspace gas NO concentration was 16.4+/-5.8 parts per million (ppm). After nitrate ingestion, gastric nitrate peaked at 20 minutes at 3,430+/-832 umol/L, plasma nitrate at 134+/-7.2 umol/L, salivary nitrate at 1516.7+/-280.5 umol/L, and salivary nitrite at 761.5+/-187.7 umol/L after 20-40 minutes. Gastric nitrite concentrations tended to be low, variable, and any rise was non-sustained. Gastric NO concentrations rose considerably from 14.8+/-3.1 ppm to 89.4+/-28.6 ppm (p < 0.0001) after 60 minutes. All parameters remained increased significantly for the duration of the study. CONCLUSIONS: A very large and sustained increase in chemically derived gastric NO concentrations after an oral nitrate load was shown, which may be important both in host defense against swallowed pathogens and in gastric physiology.
PMID:9071933 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1027050 McKnight GM et al; Gut 40 (2): 211-4 (1997)
For more Metabolism/Metabolites (Complete) data for POTASSIUM NITRATE (6 total), please visit the HSDB record page.
Potassium (K+) is the principal cation modulating the osmotic balance of the body fluids. In animals, the maintenance of normal cell volume and pressure is dependent on Na+ and K+ pumping. Potassium transport through the hydrophobic interior of a cell membrane may be facilitated by several naturally occurring compounds that form lipid-soluble alkali metal cation complexes. Potassium has the critical role of a calcium counter-ion for numerous carboxylates, phosphates, and sulfates, and also acts to stabilize macromolecular structures. Potassium is the primary agent for common, over the counter de-sensitizing toothpaste that prevents the transmission of nerve endings to the teeth. Potassium salts, including potassium nitrate, potassium chloride or potassium citrate work by diffusion across the dentinal tubules, causing depolarization of the nerve cells. In turn, these cells become unresponsive to excitatory stimuli. The effect of the potassium nitrate accumulates over time, and it may take several weeks for patients to notice improvement of pain symptoms. Potassium nitrates control pests using a unique mechanism of action. Rather than directly poisoning rodents, nitrates support the combustion of charcoal in gas cartridges, promoting the production of toxic gases, which, are lethal to the target pest. The environmental protection agency in the USA (EPA) is only minimally concerned about the risk of direct human exposure to sodium or potassium nitrates, rather than pesticide accidents--typically involving skin burns or inhalation of toxic gases.
A series of reactions is involved by which it is proposed that nitrate in water may be converted to n-nitroso cmpd that are direct carcinogenic agents. The steps in the reaction sequence are: 1. Reduction of nitrate to nitrite. 2. Reaction of nitrite with secondary amines or amides in food or water to form n-nitroso cmpd. 3. Carcinogenic reaction of n-nitroso cmpd to the extent that this series of processes actually operates in human body, nitrate has capacity to become procarcinogen. ... problem is prospective rather than realized one. /Nitrate/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 421
Acute toxicity of nitrate occurs as result of reduction to nitrite, a process that can occur under specific conditions in the stomach ... /and/ in saliva. Nitrite acts in blood to oxidize hemoglobin to methemoglobin, which does not perform as oxygen carrier to tissues ... Anoxia and death may occur. /Nitrate/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 416
Nitrates can be reduced to nitrites which can react with amines or amides and form N-nitroso cmpd (containing the group =N-N=O). N-nitroso cmpd are carcinogenic in a wide range of animal species, most are mutagenic in test systems and some have been teratogenic in animals. It is highly probable that N-nitroso cmpd also may be carcinogenic in man. Therefore exposure to N-nitroso cmpd and their precursors (nitrite, amines and amides) should be kept as low as practically achievable. Relationships have been sought between occurrence of stomach cancer and nitrate content of soil and water in Chile, Colombia and the United Kingdom, but none was established.
European Chemicals Bureau; IUCLID Dataset, Potassium nitrate (7757-79-1) (2000 CD-ROM edition). Available from, as of October 26, 2006: https://esis.jrc.ec.europa.eu/
Acute nitrate toxicity is almost always seen in infants rather than adults when it results from ingestion of well waters and vegetables high in nitrates ... /It was/ deduced that infants were prone to upset stomachs and achlorhydria. As result, stomach pH increased in alkalinity allowing nitrate-reducing organisms to enter and to reduce nitrates to nitrites. A gastric pH above 4 supports nitrate-reducing organisms ... Immature enzyme systems may also be of importance ... Fetal hemoglobin (hemoglobin F) is oxidized by nitrite to methemoglobin at rate twice as rapid as adult hemoglobin (hemoglobin A). Furthermore, enzymatic capacity of erythrocytes of newborn infants to reduce methemoglobin to hemoglobin appears less than that of adults. Difference is probably due to developmental deficiency in activity of DPNH-methemoglobin reductase (diphosphopyridine nucleotide). As opposed to adults, several clinical, physiologic and metabolic factors predispose infants to development of methemoglobinemia and acute nitrate poisoning. /Nitrate and nitrite/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 420