

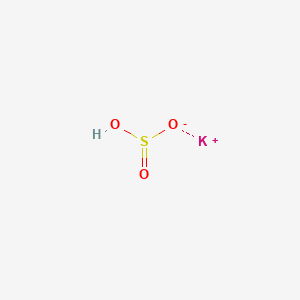

1. Potassium Hydrogen Sulfite
2. 7773-03-7
3. Potassium;hydrogen Sulfite
4. Sulfurous Acid, Potassium Salt (1:1)
5. Qjk5lo891p
6. 4429-42-9
7. Monopotassium Sulfite
8. Kaliumbisulfit
9. Potassium Acid Sulfite
10. Potassium Hydrogensulfite
11. Potassium Bisulphite
12. Potassium Hydrogen Sulphite
13. Sulfite, Potassium Metabi-
14. Unii-qjk5lo891p
15. Potassium Sulfite, Hydrogen
16. Potassium Sulfite (khso3)
17. Ins No.228
18. Dtxsid6064795
19. Potassium Bisulfite [ii]
20. Hsdb 1263
21. Ins-228
22. Potassium Bisulfite [hsdb]
23. Sulfurous Acid, Monopotassium Salt
24. Potassium Bisulfite [mart.]
25. Einecs 231-870-1
26. Akos025293926
27. E-228
28. Q412524
| Molecular Weight | 120.17 g/mol |
|---|---|
| Molecular Formula | HKO3S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 119.92834655 g/mol |
| Monoisotopic Mass | 119.92834655 g/mol |
| Topological Polar Surface Area | 79.6 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 33.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG; BETWEEN 1 OZ AND 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB). /SULFITE SALTS/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-85
FAIRLY LARGE DOSES OF SULFITES CAN BE TOLERATED SINCE THEY ARE RAPIDLY OXIDIZED TO SULFATES... /SULFITES/
Sax, N.I. Dangerous Properties of Industrial Materials. 4th ed. New York: Van Nostrand Reinhold, 1975., p. 1047
