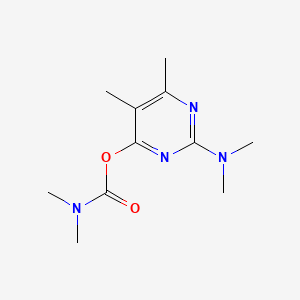

1. 2-dimethylamino-5,6-dimethylpyrimidin-4-yldimethylcarbamate
2. 5,6-dimethyl-2-dimethylamino-4-dimethylcarbamoyloxypyrimidine
3. Pirimor
4. Pyrimor
5. Zz-aphox
1. 23103-98-2
2. Pirimor
3. Pyrimor
4. Primicarbe
5. Aficida
6. Fernos
7. Aphox
8. Rapid
9. Pirimor G
10. Pirimor Granulate
11. Pirimicarb Solution
12. Pirimicarbe
13. Abol
14. 2-(dimethylamino)-5,6-dimethylpyrimidin-4-yl Dimethylcarbamate
15. Ent-27766
16. Pp 062
17. Carbamic Acid, Dimethyl-, 2-(dimethylamino)-5,6-dimethyl-4-pyrimidinyl Ester
18. 2-dimethylamino-5,6-dimethyl-4-pyrimidinyl Dimethylcarbamate
19. Dimethylcarbamic Acid 2-(dimethylamino)-5,6-dimethyl-4-pyrimidinyl Ester
20. Chebi:8248
21. 5,6-dimethyl-2-dimethylamino-4-pyrimidinyldimethylcarbamate
22. 2-(dimethylamino)-5,6-dimethyl-4-pyrimidinyldimethylcarbamate
23. 1i93ps935t
24. Pirimicarb 1000 Microg/ml In Acetone
25. Pirimicarb 10 Microg/ml In Acetonitrile
26. Pirimicarb 100 Microg/ml In Cyclohexane
27. Pirimicarb 100 Microg/ml In Acetonitrile
28. [2-(dimethylamino)-5,6-dimethylpyrimidin-4-yl] N,n-dimethylcarbamate
29. Pirimor 50 Dp
30. Caswell No. 359c
31. 2-dimethylamino-5,6-dimethylpyrimidin-4-yl Dimethylcarbamate
32. Pirimicarbe [iso-french]
33. Pirimicarb [ansi:bsi:iso]
34. Hsdb 7005
35. Einecs 245-430-1
36. Epa Pesticide Chemical Code 106101
37. Brn 0663442
38. Pyrimicarbe
39. Unii-1i93ps935t
40. Ai3-27766
41. Pirimicarb [mi]
42. Pirimicarb [iso]
43. Pirimicarb [hsdb]
44. 5,6-dwumetylo-2-dwumetyloamino-4-pirimidynylodwukarbaminian [polish]
45. Dsstox_cid_12569
46. Dsstox_rid_78989
47. Dsstox_gsid_32569
48. Schembl26523
49. 5-25-13-00063 (beilstein Handbook Reference)
50. Chembl1870931
51. Dtxsid1032569
52. Yfgyufniohwbob-uhfffaoysa-
53. 5,6-dwumetylo-2-dwumetyloamino-4-pirimidynylodwukarbaminian
54. 5,6-dimethyl-2-dimethylamino-4-dimethylcarbamoyloxypyrimidine
55. Zinc900772
56. 2-(dimethylamino)-5,6-dimethyl-4-pyrimidinyl Dimethylcarbamate
57. Oms 1330
58. Tox21_301003
59. 2-(dimethylamino)-5,6-dimethyl-4-pyrimidinyl N,n-dimethylcarbamate
60. Mfcd00055520
61. Akos015892522
62. Pirimicarb 100 Microg/ml In Methanol
63. Gs-3028
64. Ncgc00163704-01
65. Ncgc00163704-02
66. Ncgc00163704-03
67. Ncgc00254905-01
68. Cas-23103-98-2
69. Db-103794
70. Hy-119419
71. Cs-0068146
72. Ft-0655935
73. Pirimicarb, Pestanal(r), Analytical Standard
74. H10475
75. 103p982
76. A816567
77. Q419705
78. J-014990
79. (2-dimethylamino-5,6-dimethylpyrimidin-4-yl) N,n-dimethylcarbamate
80. 2-(dimethylamino)-5,6-dimethylpyrimidin-4-yl N,n-dimethylcarbamate
81. 2-dimethylamino-5,6-dimethylpyrimidin-4-yl N,n-dimethylcarbamate
82. [2-(dimethylamino)-5,6-dimethyl-pyrimidin-4-yl] N,n-dimethylcarbamate
83. Carbamic Acid, Dimethyl-, 2-(dimethylamino)-5,6-dimethyl-4-pyrimidyl Ester
84. Dimethyl-carbamic Acid 2-dimethylamino-5,6-dimethyl-pyrimidin-4-yl Ester
| Molecular Weight | 238.29 g/mol |
|---|---|
| Molecular Formula | C11H18N4O2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 238.14297583 g/mol |
| Monoisotopic Mass | 238.14297583 g/mol |
| Topological Polar Surface Area | 58.6 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
In rats given carbonyl-labeled [14C]pirimicarb by gavage or ip injection, >50% of the dose was expired as 14CO2 within 5 hr, & 15% was eliminated in the urine; essentially no residues were detected at sacrifice 8 days after admin. Up to 4 daily admins of pirimicarb to rats resulted in no accumulation in adipose tissue. In dogs, 86-94% of a ring-14C-labeled dose of pirimicarb was recovered, 79-88% in the urine & 6-7% in the feces; recovery was 74-86% after 1 day. Of a carbonyl-labeled dose to dogs, 15-26% was recovered, primarily in the urine; the unrecovered portion was thought to be expired rapidly as 14CO2. Of pirimicarb admin to a lactating dairy cow, 96% of the dose appeared in the urine, 4% in the feces, & <0.3% in the milk.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1170
Mode of action: Selective systemic insecticide with contact, stomach, and respiratory action. Absorbed by the roots, and translocated through the xylem. Penetrates the leaves, but is not translocated extensively.
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 820
Pirimicarb's major urinary metabolites in rats, dogs, & cows were similar, resulting from oxidative & hydrolytic mechanisms & consisting primarily of hydroxypyrimidines with modifications of the alkyl constituents of the heterocyclic moiety. Of the administered dose, 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine accounted for 10-16.3%, 2-methylamino-5,6-dimethyl-4-hydroxypyrimidine for 20.5-41%, 2-amino-5,6-dimethyl-4-hydroxypyrimidine for 12.9-21%, & 2-dimethylamino-6-hydroxymethyl-5-methyl-4-hydroxypyrimidine for 1.8-5.7%. The major metabolites were eliminated unconjugated.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1170
... During ... metabolism /of pirimicarb/ in mammals the carbamate moiety is hydrolyzed & subsequent demethylation at the dimethylamino group which is attached to the heterocyclic moiety results in the following major metabolites which are excreted in urine: 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine (DDHP), 2-methylamino-5,6-dimethyl-4-hydroxypyrimidine (MDHP), & 2-amino-5,6-dimethyl-4-hydroxypyrimidine (ADHP). These metabolites were detected in every urine sample of seven workers who had applied pirimicarb. Concns of the 2-methylamino-5,6-dimethyl-4-hydroxypyrimidine & 2-amino-5,6-dimethyl-4-hydroxypyrimidine were much higher than that of 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine indicating a considerable demethylation capacity in humans. No metabolites were found in urine specimens of controls. The investigated pyrimidines represent sensitive & specific parameters for biological monitoring of exposure to pirimicarb.
PMID:10414785 Hardt J, et al; Toxicol Lett 107 (1-3): 89-93 (1999)
Cholinesterase inhibitor.
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 820
