


1. Anaprilin
2. Anapriline
3. Avlocardyl
4. Ay 20694
5. Ay-20694
6. Ay20694
7. Betadren
8. Dociton
9. Hydrochloride, Propranolol
10. Inderal
11. Obsidan
12. Obzidan
13. Propanolol
14. Propranolol
15. Propranolol Hydrochloride
16. Rexigen
1. 5051-22-9
2. R-(+)-propranolol
3. (+)-propranolol
4. D-propranolol
5. Dextropropranolol
6. 2r-propranolol
7. (r)-(+)-propranolol
8. (r)-propranolol
9. R (+)-propanolol
10. Dexpropranololum
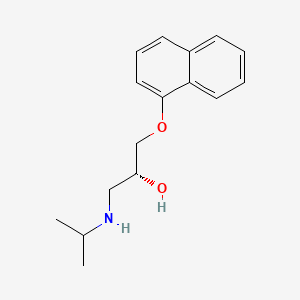
11. (+)-1-isopropylamino-3-(1-naphthyloxy)-2-propanol
12. R(+)-propranolol
13. (2r)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol
14. Propranolol, (r)-
15. Pg6ky07ud7
16. Chebi:8736
17. 2-propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, (+)-
18. Ncgc00016429-05
19. 2-propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, (r)-
20. 2-propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, (2r)-
21. 2-propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, (r)-
22. Dsstox_cid_25304
23. Dsstox_rid_80790
24. Dsstox_gsid_45304
25. Despropranolo [dcit]
26. Despropranolo
27. (r)-1-(isopropylamino)-3-(naphthalen-1-yloxy)propan-2-ol
28. (2r)-1-(naphthalen-1-yloxy)-3-[(propan-2-yl)amino]propan-2-ol
29. (2r)-3-(naphthalen-1-yloxy)-1-(propan-2-ylamino)propan-2-ol
30. Mls001333595
31. Dexpropranolol [inn:ban]
32. Dexpropranololum [inn-latin]
33. Cas-5051-22-9
34. Smr000875288
35. Einecs 225-749-2
36. Unii-pg6ky07ud7
37. R-propanolol
38. Propranolol-(r)
39. Rnp
40. D-(+)-propranolol
41. (r)-(+)-propanolol Hydrochloride
42. (2r)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol;hydrochloride
43. Tocris-0624
44. Tocris-0835
45. (+)-(r)-propranolol
46. Prestwick0_001075
47. Prestwick1_001075
48. Prestwick2_001075
49. Lopac-p-0884
50. Lopac-p-8688
51. Cas-318-98-9
52. Dexpropranolol [inn]
53. Schembl25255
54. Cid_66366
55. Bidd:gt0130
56. Spbio_002995
57. Chembl275742
58. Gtpl7596
59. Dtxsid3045304
60. Bdbm60973
61. Zinc20240
62. Tox21_110435
63. Mfcd00066275
64. Pdsp1_000775
65. Pdsp1_001089
66. Pdsp2_000763
67. Pdsp2_001073
68. Akos025212355
69. Tox21_110435_1
70. Cas-839971
71. Db03322
72. Ncgc00015798-01
73. Ncgc00015798-02
74. Ncgc00015798-03
75. Ncgc00015798-11
76. Ncgc00016429-01
77. Ncgc00016429-02
78. Ncgc00016429-03
79. Ncgc00016429-04
80. Ncgc00016429-06
81. Ncgc00024690-01
82. Ncgc00024814-01
83. Ncgc00024814-02
84. Cas-13071-11-9
85. Ab00514691
86. Brd-k92830582-003-04-8
87. Q27088458
88. (+)-1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol
89. (2r)-1-(isopropylamino)-3-(1-naphthyloxy)propan-2-ol
90. 1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol, (r)-
91. (2r)-1-(isopropylamino)-3-(1-naphthoxy)propan-2-ol;hydrochloride
92. (2r)-1-(1-naphthalenyloxy)-3-(propan-2-ylamino)-2-propanol;hydrochloride
| Molecular Weight | 259.34 g/mol |
|---|---|
| Molecular Formula | C16H21NO2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 259.157228913 g/mol |
| Monoisotopic Mass | 259.157228913 g/mol |
| Topological Polar Surface Area | 41.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 257 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic beta-Antagonists
Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Dexpropranolol (propranolol) has known human metabolites that include 4-Hydroxypropranolol, 5-Hydroxypropranolol, and N-Desisopropylpropranolol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
