

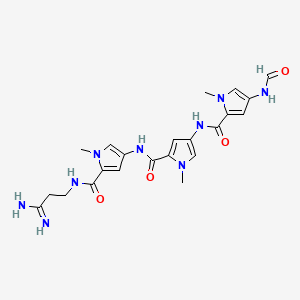

1. Distamycin
2. Distamycin A
3. Stallimycin Hydrochloride
4. Stallimycin Monohydrochloride
1. Distamycin A
2. Distamycin
3. 636-47-5
4. Herperetin
5. Stallimycin [inn]
6. Dst-a
7. Nsc82150
8. Nsc-82150
9. Fi 6426
10. Chembl11252
11. 80o63p88is
12. N-[5-[[5-[(3-amino-3-iminopropyl)carbamoyl]-1-methylpyrrol-3-yl]carbamoyl]-1-methylpyrrol-3-yl]-4-formamido-1-methylpyrrole-2-carboxamide
13. Ncgc00018292-04
14. Distamycin 3
15. Nsc 150528
16. Estalimicina
17. Stallimicina
18. Stallimycine
19. Stallimycinum
20. Distamicina A
21. Distamycin Hydrochloride
22. 1h-pyrrole-2-carboxamide, N-(5-(((3-amino-3-iminopropyl)amino)carbonyl)-1-methyl-1h-pyrrol-3-yl)-4-(((4-(formylamino)-1-methyl-1h-pyrrol-2-yl)carbonyl)amino)-1-methyl-
23. 1h-pyrrole-2-carboxamide, N-[5-[[(3-amino-3-iminopropyl)amino]carbonyl]-1-methyl-1h-pyrrol-3-yl]-4-[[[4-(formylamino)-1-methyl-1h-pyrrol-2-yl]carbonyl]amino]-1-methyl-
24. Nsc150528
25. Stallimicina [dcit]
26. Distamicina A [italian]
27. Stallimycine [inn-french]
28. Stallimycinum [inn-latin]
29. Estalimicina [inn-spanish]
30. Unii-80o63p88is
31. Nsc 82150
32. Brn 0468437
33. Dst-3
34. Distamycin A [mi]
35. Dsstox_cid_25637
36. Dsstox_rid_81018
37. Dsstox_gsid_45637
38. Distamycin; Stallimycin
39. Schembl108585
40. Dtxsid9045637
41. Cid_6602691
42. Zinc3872327
43. Tox21_110857
44. Bdbm50055659
45. Ccg-36393
46. N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-n-4':n'-4''-ter-(2-pyrrolcarboxamid)
47. Ncgc00018292-01
48. Ncgc00018292-02
49. Ncgc00018292-03
50. Ncgc00018292-05
51. Ncgc00024382-03
52. Cas-636-47-5
53. Nci60_041805
54. Sr-01000000269
55. Sr-01000000269-3
56. Q27269162
57. N,4''-ter[pyrrole-2-carboxamide], N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-
58. (n,4':n',4''-terpyrrole)-2-carboxamide, N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-
59. 2n-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-1h-2-pyrrolylcarboxamido]-1-methyl-1h-2-pyrrolecarboxamide
60. 2n-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1h-3-pyrrolyl]-4-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-1h-2-pyrrolecarboxamide
61. 2n-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1h-3-pyrrolyl]-4-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-1h-2-pyrrolecarboxamide(distamycin A)
62. 2n-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1h-3-pyrrolyl]-4-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-1h-2-pyrrolecarboxamide(distamycin)
63. 39389-47-4
64. 4n-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1h-2-pyrrolyl]-2-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-1h-4-pyrrolecarboxamide
65. 5n-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-2,3-dihydro-1h-3-pyrrolyl]-3-(4-formamido-1-methyl-1h-2-pyrrolylcarboxamido)-1-methyl-2,3-dihydro-1h-5-pyrrolecarboxamide
66. N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-n,4':n',4''-ter(pyrrole-2-carboxamide)
67. N-(3-amino-3-iminopropyl)-4-(4-(4-formamido-1-methyl-1h-pyrrole-2-carboxamido)-1-methyl-1h-pyrrole-2-carboxamido)-1-methyl-1h-pyrrole-2-carboxamide
68. N-[5-({5-[(3-amino-3-iminopropyl)carbamoyl]-1-methyl-1h-pyrrol-3-yl}carbamoyl)-1-methyl-1h-pyrrol-3-yl]-4-(formylamino)-1-methyl-1h-pyrrole-2-carboxamide
69. N-[5-[[(3-amino-3-iminopropyl)amino]carbonyl]-1-methyl-1h-pyrrol-3-yl]-4-[[[4-(formylamino)-methyl-1h-pyrrol-2-yl]carbonyl]amino]-1h-pyrrole-2-carboxamide
70. N-[5-[[(amino-3-iminopropyl)amino]carbonyl]-1-methyl-1h-pyrrol-3-yl-4-[[(4-(formylamino)-1-methyl-1h-pyrrol-2yl]carbonyl]amino]-1h-pyrrole-2-carboxamide
71. N-[5-[[5-[(3-amino-3-imino-propyl)carbamoyl]-1-methyl-pyrrol-3-yl]carbamoyl]-1-methyl-pyrrol-3-yl]-4-formamido-1-methyl-pyrrole-2-carboxamide
| Molecular Weight | 481.5 g/mol |
|---|---|
| Molecular Formula | C22H27N9O4 |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 481.21860038 g/mol |
| Monoisotopic Mass | 481.21860038 g/mol |
| Topological Polar Surface Area | 181 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 825 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
